Potassium telluride
 K+: __ Te2-: __
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.039 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| K2Te | |
| Molar mass | 298.64 g/mol |
| Appearance | pale yellow powder, turns grey when exposed to air[1] |
| Melting point | 874 °C |
| Related compounds | |
Other anions
|
Potassium oxide Potassium sulfide Potassium selenide Potassium polonide |
Other cations
|
Lithium telluride Sodium telluride Rubidium telluride Caesium telluride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
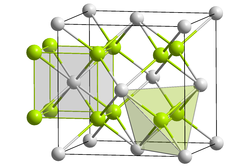
Potassium telluride is an inorganic compound with a chemical formula K2Te. It is formed from potassium and tellurium, making it a telluride.[2] Potassium telluride is a white powder. Like rubidium telluride and caesium telluride, it can be used as an ultraviolet detector in space. Its crystal structure is similar to other tellurides, which have an anti-fluorite structure.
Production[edit]
Tellurium will react with melting potassium cyanide (KCN) producing potassium telluride. It can also be produced by direct combination of potassium and tellurium, usually in liquid ammonia solvent:[3]
Reactions[edit]
Adding potassium telluride to water and letting the filtrate stand in air leads to an oxidation reaction that generates potassium hydroxide (KOH) and elemental tellurium:[3][4]
References[edit]
- Sangester J. and Pelton AD; Journal of Phase Equilibria, 1997, 18(4) p. 394.
- ^ Linda D. Schultz (October 1990). "Synthesis and characterization of potassium polytellurides in liquid ammonia solution". Inorganica Chimica Acta. 176 (2): 271–275. doi:10.1016/S0020-1693(00)84855-0. Retrieved 2019-11-23.
- ^ Brigitte Eisenmann, Herbert Schäfer: K2Te3 : The First Binary Alkali-Metal Polytelluride with Te2−3-Ions. In: Angewandte Chemie International Edition in English. 17, 1978, S. 684, doi:10.1002/anie.197806841.
- ^ a b Wolfgang A. Herrmann, Christian Erich Zybill (2014). Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 4, 1997 Volume 4: Sulfur, Selenium and Tellurium. Georg Thieme Verlag. p. 191. ISBN 978-3-13-179191-7.
- ^ Adolf Pinner (1885), Repetitorium Der Anorganischen Chemie [Repetitorium of Inorganic Chemistry] (in German), Рипол Классик, p. 116, ISBN 978-5-87746-719-4


