User:Clairette3393/sandbox
SMAD 4, also called SMAD family member n°4 or Mothers against decapentaplegic homolog 4, is a protein involved in cell signaling in mammals. It belongs to the proteins of the SMAD family.
SMAD 4 participates in chain reactions inside cells where it interacts with other members of the SMAD family such as SMAD2 or SMAD3. SMAD 4 forms with SMAD 3 a complex which can bind to DNA and modify the expression of several genes related to cellular activities such as proliferation or differentiation [1]. The complex SMAD 3-SMAD 4 is therefore a transcription factor.
Intracellular reactions involving SMAD 4 are triggered by the biding, on the surface of the cells, of growth factors from the TGFβ family. The sequence of intracellular reactions involving SMADS is called the SMAD pathway or the transforming growth factor beta (TGF-β) pathway, since the sequence starts with the recognition of TGF-β by cells.
SMAD 4 belongs to the co-SMAD group, the second class of the SMAD family. SMAD 4 is the only known co-SMAD in mammals.
It also belongs to the Darwin family of proteins that modulate members of the TGFβ superfamily, a family of proteins that all play a role in the regulation of cellular responses.
Mammalian SMAD 4 is a homolog of the Drosophila protein Mothers against decapentaplegic or MAD.
Protein structure and nomenclature[edit]
Structure of SMAD 4[edit]
SMAD family member 4 is a 552 amino-acid polypeptide with a molecular weight of 60 439 daltons. This protein is composed of only one chain of amino acids.
SMAD 4 is always coded in mammals by a gene located on chromosome 18. In humans, the protein is coded by the SMAD 4 gene, which is located in the region 21.1 of chromosome 18 (ref 1) [2] The human gene contains 54 829 base pairs and is located from pair n° 51,030,212 to pair 51,085,041. [3]

SMAD 4 has a tri-dimensional structure and two functional domains known as MH1 and MH2. The letters M and H stand for MAD Homology and refer to the similarity between mammals SMAD 4 and the Drosophilia protein Mothers against decapentaplegic (MAD).(ref phosphorylation of threonine).
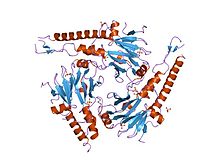
The MH1 domain, corresponding to the N-terminus, contains amino-acids with physical and chemical properties that give SMAD 4 specific DNA-binding properties. This region recognizes a specific DNA sequence composed of eight nucleotides: G-T-C-T-A-G-A-C.
[4]
The sequence is also recognized by SMAD 3, which forms a protein complex with SMAD 4 in the cytoplasm of mammalian cells. It is therefore sometimes called the "consensus sequence" but is mostly known as Smad-binding DNA element (SBE). The MH1 domain facilitates the binding of SMAD 4 to receptors such as SMAD 2 and SMAD 3 which belong to the R-SMAD group (first class of the SMAD family) by interacting with their phosphorylated MH2 domain.
The MH2 domain, corresponding to the C-terminus, is responsible for receptor recognition, DNA binding and association with other SMADs. Just as the MH1 domain interacts with the R-SMADS MH2 domain, the SMAD 4 MH2 domain directly interacts with their MH1 domain.[3] SMAD 4 can therefore form with other SMADS heterodimers and heterotrimers.
Nomenclature: origin and alternative names[edit]
SMADs are highly conserved across species, especially in the N terminal MH1 domain and the C terminal MH2 domain. The SMAD proteins are homologs of both the Drosophila protein MAD and the C. elegans protein SMA. The name is a combination of the two. During Drosophila research, it was found that a mutation in the gene MAD in the mother repressed the gene decapentaplegic in the embryo. The phrase "Mothers against" was added, since mothers often form organizations opposing various issues, e.g. Mothers Against Drunk Driving (MADD), reflecting "the maternal-effect enhancement of dpp".[2]
SMAD 4 is also known as DPC4, JIP or MADH4. [5]
Function and action mechanism[edit]
SMAD 4 is a protein defined as an essential effector in the SMAD pathway. SMAD 4 serves as a mediator between extracellular growth factors from the TGFβ family and genes inside the cell nucleus. The abbreviation co in co-SMAD stands for common mediator. SMAD 4 is also defined as a signal transducer.
In the TGF-β pathway, TGF-β dimers are recognized by a a transmembrane receptor, known as type II receptor. Once the type II receptor is activated by the binding of TGF-β, it phosphorylates a type I receptor. Type I receptor is also a cell surface receptor. This receptor then phosphorylates receptor regulated SMADS (R-SMAD) such as SMAD 2 or SMAD 3. The phosphorylated R-SMADS then bind to SMAD 4. The R-SMADs-SMAD 4 association is a heteromeric complex. This complex is going to move from the cytoplasm to the nucleus: this is the translocation. SMAD4 may form heterotrimeric, heterohexameric or heterodimeric complexes with R-SMADS.
In the nucleus the heteromeric complex binds promoters and interact with transcriptional activators. SMAD3/SMAD4 complexes can directly bind the SBE. These associations are weak and require additional transcription factors such as members of the AP-1 family, TFE3 and FoxG1 to regulate gene expression.[6]
Many TGFβ ligands use this pathway and subsequently SMAD4 is involved in many cell functions such as differentiation, apoptosis, gastrulation, embryonic development and the cell cycle.
[edit]
Genetic experiments such as gene knockout (KO), which consist in modifying or inactivating a gene, can be carried out in order to see the effects of a dysfunctional SMAD 4 on the study organism. Experiments are often conducted in the house mouse (Mus musculus).
It has been shown that, in mice KO of SMAD4, the granulosa cells, which secrete hormones and growth factors during the oocyte development, undergo premature luteinisation and express lower levels of follicle-stimulating hormone receptors (FSHR) and higher levels of luteinizing hormone receptors (LHR). This may be due in part to impairment of bone morphogenetic protein-7 effects as BMP-7 uses the SMAD 4 signaling pathway.[5][6]
Deletions in the genes coding for SMAD1 and SMAD5 have also been linked to metastasic granulosa cell tumors in mice.[7]
SMAD4 is often found to be mutated in many cancers. The mutation can be inherited or acquired during an individual’s lifetime. If inherited, the mutation affects both somatic and sexual cells. If the SMAD 4 mutation is acquired, it will only exist in certain somatic cells.(ref) Indeed, SMAD 4 is not synthesized by all cells. The protein is present in skin, pancreatic, colon, uterus and epithelial cells. It is also produced by fibroblasts.
The functional SMAD 4 participates to the regulation of the TGFβ pathway, which negatively regulates growth of epithelial cells and the extracellular matrix (ECM). When the structure of SMAD 4 is altered, expression of the genes involved in cell growth is no longer regulated and cell proliferation can go on without any inhibition. The important number of cell divisions leads to the forming of tumors and then to multiploid colorectal cancer and pancreatic carcinoma. SMAD 4 is found inactivated in at least 50% of pancreatic cancers.[7]
Somatic mutations of the MH1 domain found in human cancers have been shown to inhibit the DNA-binding function of this domain.
SMAD 4 is also found mutated in the autosomal dominant disease juvenile polyposis syndrome (JPS). JPS is characterized by hamartomatous polyps in the gastrointestinal (GI) tract. These polyps are usually benign, however they are at greater risk of developing gastrointestinalcancers, in particular colon cancer. Around 60 mutations causing JPS have been identified. They have been linked to the production of a smaller SMAD 4, with missing domains that prevent the protein from binding to R-SMADS and forming heteromeric complexes. [8]
Mutations in SMAD 4 (mostly susbtitutions), can cause Myhre syndrome, a rare inherited disorder characterized by mental disabilities, short stature, unusual facial features, and various bone abnormalities. [9]
References[edit]
- ^ Feng XH, Lin X, Liang M, Liang YY, Brunicardi FC, Melchior F (March 2003)."SUMO-1 modification in Smad 4/DPC4", The Journal of Biological Chemistry: JBC Papers in Press, Manuscript M302243200, 2-23, doi: 10.1074/jbc.M302243200
- ^ SMAD 4, The Genetics Home Reference Website,US National Library of Medecine. http://ghr.nlm.nih.gov/gene/SMAD4
- ^ SMAD 4, The Genetics Home Reference Website,US National Library of Medecine. http://ghr.nlm.nih.gov/gene/SMAD4
- ^ Zawel L, Le Dai J, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE (March 1998). "Human Smad3 and Smad4 Are Sequence-Specific Transcription Activators", Molecular Cell, Vol 1(4), 611-617, PMID: 9660945
- ^ SMAD 4, The Genetics Home Reference Website,US National Library of Medecine. http://ghr.nlm.nih.gov/gene/SMAD4
- ^ Cite error: The named reference
pmid15702493was invoked but never defined (see the help page). - ^ Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA (December 2009)."Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors", Endocrinology, 150(12):5208-17. doi: 10.1210/en.2009-0644
- ^ SMAD 4, The Genetics Home Reference Website,US National Library of Medecine. http://ghr.nlm.nih.gov/gene/SMAD4
- ^ Growth-Mental Deficiency Syndrome of Myhre, National Organization for rare disorders. http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/1075/viewFullReport
External Links[edit]
[[ http://water.epa.gov/drink/contaminants/basicinformation/pentachlorophenol.cfm |US EPA: Pentachrorophenol in drinking water ]]

