User:Drealle/Sandbox
| Drealle/Sandbox |
|---|
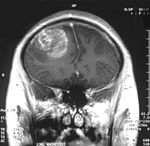


Glioblastoma multiforme (GBM) is the most common and aggressive type of primary brain tumor, accounting for 52% of all primary brain tumor cases and 20% of all intracranial tumors. Despite being the most prevalent form of primary brain tumor, GBMs occur in only 2–3 cases per 100,000 people in Europe and North America.
Treatment can involve chemotherapy, radiotherapy, and surgery, all of which are acknowledged as palliative measures, meaning that they do not provide a cure. Even with complete surgical resection of the tumor, combined with the best available treatment, the survival rate for GBM remains very low. However, many advances in microsurgery techniques, radiotherapy and chemotherapy are slowly increasing the survival time of patients diagnosed with glioblastoma.
Glioblastoma multiform can be divided into three subgroups dependent on Karnofsky Performance Score (KPS), the age of the patient, and treatment:[1]
| RPA class | Definition | Historical Median Survival Time | Historical 1-Year Survival | Historical 3-Year Survival | Historical 5-Year Survival |
|---|---|---|---|---|---|
| III | Age <50, KPS >= 90 | 17.1 months | 70% | 20% | 14% |
| IV | Age < 50, KPS < 90 | 11.2 months | 46% | 7% | 4% |
| Age > 50, KPS >= 70, surgical removal with good neurologic function | |||||
| V + VI | Age >= 50, KPS >= 70, surgical removal with poor neurologic function | 7.5 months | 28% | 1% | 0% |
| Age >= 50, KPS >= 70, no surgical removal | |||||
| Age >= 50, KPS < 70 |
Causes[edit]
GBM is more common in males, although the reason for this is not clear [2]. Most glioblastoma tumors appear to be sporadic, without any genetic predisposition. No links have been found between glioblastoma and smoking[3], diet[4], cellular phones[5], electromagnetic fields[6], or viral infection[7]. There appears to be a small link between ionizing radiation and glioblastoma[8].
Pathogenesis[edit]
Glioblastomas multiforme are characterized by the presence of small areas of necrotizing tissue that is surrounded by highly-anaplastic cells (pseudopalisading necrosis). This characteristic, as well as the presence of hyperplastic blood vessels, differentiates the tumor from Grade 3 astrocytomas, which do not have these features. Although glioblastoma multiforme can be formed from lower-grade astrocytomas, post-mortem autopsies have revealed that most glioblastomas multiforme are not caused by previous lesions in the brain.
Unlike oligodendrogliomas, glioblastomas multiforme can form in either the gray matter or the white matter of the brain; but most GBM arises from the deep white matter and quickly infiltrate the brain, often becoming very large before producing symptoms. The tumor may extend to the meningeal or ventricular wall, leading to the high protein content of cerebrospinal fluid (CSF) (> 100 mg/dL), as well as an occasional pleocytosis of 10 to 100 cells, mostly lymphocytes. Malignant cells carried in the CSF may spread to the spinal cord or cause meningeal gliomatosis. However, metastasis of GBM beyond the central nervous system is extremely rare. About 50% of GBM occupy more than one lobe of a hemisphere or are bilateral. Tumors of this type usually arise from the cerebrum and may exhibit the classic infiltrate across the corpus callosum, producing a butterfly (bilateral) glioma.
The tumor may take on a variety of appearances, depending on the amount of hemorrhage, necrosis, or its age. A CT scan will usually show a nonhomogeneous mass with a hypointense center and a variable ring of enhancement surrounded by edema. Part of a lateral ventricle is usually deformed, and both lateral and third ventricles may be displaced.
Symptoms[edit]
Although common symptoms of the disease include seizure, nausea and vomiting, headache, and hemiparesis, the single most prevalent symptom is a progressive memory, personality, or neurological deficit due to temporal and frontal lobe involvement. The kind of symptoms produced depends highly on the location of the tumor, more so than on its pathological properties. The tumor can start producing symptoms quickly, but occasionally is asymptomatic until it reaches an enormous size. See Symptoms section in:
Diagnosis[edit]
In early stages, when viewed with MRI, glioblastoma may mimic more benign brain lesions[9]. Diagnosis of a suspected GBM on CT or MRI should rest on a stereotactic biopsy or by a craniotomy, which can, at the same time, remove as much tumor as possible. Although the entire tumor can never be removed, in theory due to its multicentricity and diffuse character, partial resection ("debulking") can still prolong survival slightly.
Treatment[edit]
It is very difficult to treat glioblastoma due to several complicating factors:[10]
- The tumor cells are very resistant to chemotherapy and other conventional therapies
- The brain is susceptible to damage due to therapy
- The brain has a very limited capacity to repair itself
- Many drugs cannot cross the blood brain barrier to act on the tumor
Treatment of primary brain tumors and brain metastases consists of both symptomatic and palliative therapies.
Symptomatic therapy[edit]
Supportive treatment focuses on relieving symptoms and improving the patient’s neurologic function. The primary supportive agents are anticonvulsants and corticosteroids.
- Historically, around ninety percent of patients with glioblastoma underwent anticonvulsant treatment, although is has been estimated that only approximately 40% of patients required this treatment. Recently, it has not been recommended that neurosurgeons administer anticonvulsants prophylactically, and should wait until a seizure occurs before prescribing this medication[11]. Those receiving phenytoin concurrent with radiation may have serious skin reactions such as erythema multiforme and Stevens–Johnson syndrome.
- Corticosteroids, usually dexamethasone given 4 to 10 mg every 4 to 6 h, can reduce peritumoral edema (through rearrangement of the blood-brain barrier), diminishing mass effect and lowering intracranial pressure, with a decrease in headache or drowsiness.
Palliative therapy[edit]
Palliative treatment usually is conducted to improve quality of life and to achieve a longer survival time. It includes surgery, radiation therapy, and chemotherapy. A maximally feasible resection with maximal tumor-free margins ("debulking") is usually performed along with external beam radiation and chemotherapy.
Surgery[edit]
Surgery is the first stage of treatment of glioblastoma. An average GBM tumor contains 1011 cells, which is on average reduced to 109 cells after surgery. It is used to take a section for diagnosis, to remove some of the symptoms of a large mass pressing against the brain, to remove disease before secondary resistance to radiotherapy and chemotherapy, and to prolong survival.
The greater the extent of tumor removal, the longer the survival time. Removal of 98% or more of the tumor has been associated with a significantly longer median survival time than if less than 98% of the tumor is removed[12]. The chances of near-complete initial removal of the tumor can be greatly increased if the surgery is guided by a fluorescent dye known as 5-aminolevulinic acid[13].
Radiotherapy[edit]
On average, radiotherapy after surgery can reduce the tumor size to 107 cells. Whole brain radiotherapy does not improve survival when compared to the more precise and targeted three-dimensional conformal radiotherapy[14]. A total radiation dose of 60-65 Gy has been found to be optimal for treatment [15].
Chemotherapy[edit]
The standard of care for glioblastoma includes chemotherapy during and after radiotherapy. On average, chemotherapy after surgery and radiotherapy can initially reduce the tumor size to 106 cells. The use of temozolomide both during radiotherapy and for six months post radiotherapy results in a significant increase in median survival with minimal additional toxicity[16]. This treatment regime is now standard for most cases of glioblastoma where the patient is not enrolled in a clinical trial[17][18].
Clinical trials[edit]
There are hundreds of clinical trials underway to assess the ability of new drugs and strategies to treat glioblastoma and extend the duration of survival. Below are some studies that have published promising results that have extended the survival of patients with GBM.
Pre-clinical studies[edit]
- A possible therapy technique is to use viruses to attack the cancer. A modified herpes simplex virus has been engineered to invade tumor cells, and shows some efficacy in mice[19].
- Dichloroacetate suppressed the metabolism of a glioblastoma cell line, and rats injected with a human lung cancer cell and given dichloroacetate in their drinking water had smaller tumors than the rats that did not drink dichloroacetate[20].
Phase I[edit]
- Glioblastoma patients treated with motexafin gadolinium had a median survival time that was greater than a comparison group receiving standard care[21].
- In recent studies, the antimalarial drug chloroquine has been shown to increase mid-term survival when given in combination with conventional therapy, although this difference did not reach statistical significance due to small sample size[22][23][24].
- The use of injecting magnetic nanoparticles into the tumors of patients with recurrent GBM has been shown to be safe and feasible and resulted in promising survival rates.[25]. Promising survival rates have been observed in animal models[26], however, as yet there are not any results from efficacy studies in humans.
Phase II[edit]
- Increased intracavity treatment with the radiopharmaceutical Chlorotoxin-linked-131iodine (131-I-TM-601) has been associated with increased survival of GBM patients[27].
- Intracavity treatment with Iodine131 mouse 81C6 monoclonal antibody improves survival of patients recently diagnosed with glioblastoma[28].
- Microinfusion with the antisense oligonucleotide AP 12009 into the tumor site resulted in a median survival time that exceeded published baselines with conventional chemotherapy[29].
- Patients receiving the radiosensitizer RSR-13 (efaproxiral) have demonstrated a slight increase in median survival, although supplemental oxygen was required during treatment due to hypoxia[30].
- Patients with recurrent glioblastoma who received a combination of bevacizumab and irinotecan showed an improvement in progression-free survival compared to historical survival data[31].
Phase III[edit]
- Implantation of BiCNU-impregnated wafers - trade name Gliadel - at the time of primary resection, improved median survival and prolonged the time until tumor recurrence, compared with placebo wafers[10].
- Treatment of patients with newly-diagnosed glioblastoma multiforme with photodynamic therapy as an adjuvent treatment has been associated with better prognosis and improved KPS[32][33].
Recurrences[edit]
Long-term disease-free survival is unlikely, and the tumor will often reappear, usually within 2 cm of the original site, and 10% may develop new lesions at distant sites. More extensive surgery and intense local treatment after recurrence has been associated with improved survival.[34].
Prognosis[edit]
The median survival time from the time of diagnosis without any treatment is 3 months. Increasing age (> 60 years of age) carries a worse prognostic risk. Death is usually due to cerebral edema or increased intracranial pressure. One in twenty of glioblastoma patients survive for more than three years, and approximately one in 5,000 glioblastoma patients survive for decades[35]. Ben Williams has now survived glioblastoma for more than twelve years[36].
Survival of more than three years has been associated with younger age at diagnosis, a good initial KPS, and MGMT methylation [35]. A DNA test can be conducted on glioblastomas to determine whether or not the promoter of the MGMT gene is methylated. Patients with a methylated MGMT promoter have been associated with significantly greater long-term survival than patients with an unmethylated MGMT promoter[37]. This DNA characteristic is intrinsic to the patient and currently cannot be altered externally.
Long-term survival has also been associated with those patients who receive surgery, radiotherapy, and temozolomide chemotherapy[35]. However, much remains unknown about why some patients survive longer with glioblastoma.
References[edit]
- ^ Shawl, EG, Seiferheld, W, Scott, C; et al. (2003). "Re-examining the radiation therapy oncology group (RTOG) recursive partitioning analysis (RPA) for glioblastoma multiforme (GBM) patients". International Journal of Radiation Oncology*Biology*Physics. 57 (2): S135–S136. doi:10.1016/S0360-3016(03)00843-5. PMID 15758009.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Ohgaki H, Kleihues P (2005). "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas". J Neuropathol Exp Neurol. 64: 479–89. PMID 15977639.
- ^ Zheng, T, Cantor KP, Zhang Y; et al. (2001). "Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa". Cancer Epidemiol Biomarkers Prev. 10: 413–4. PMID 11319186.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Huncharek M, Kupelnick B, Wheeler L (2003). "Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies". J Environ Pathol Toxicol Oncol. 22: 129–37. PMID 14533876.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Inskip PD, Tarone RE, Hatch EE; et al. (2001). "Cellular-telephone use and brain tumors". N Engl J Med. 344: 79–86. PMID 11150357.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Savitz DA, Checkoway H, Loomis DP (1998). "Magnetic field exposure and neurodegenerative disease mortality among electric utility workers". Epidemiology. 9: 398–404. PMID 9647903.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vilchez RA, Kozinetz CA, Arrington AS; et al. (2003). "Simian virus 40 in human cancers". Am J Med. 114: 675–84. doi:10.1016/S0002-9343(03)00087-1. PMID 12798456.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Cavenee L (2000). "High-grade gliomas with chromosome 1p loss". J Neurosurg. 92: 1080–1. PMID 10839286.
- ^ Jung TY, Jung S (2007). "Early neuroimaging findings of glioblastoma mimicking non-neoplastic cerebral lesion". Neurol Med Chir (Tokyo). 47 (9): 424–7. PMID 17895617.
- ^ a b
Lawson HC, Sampath P, Bohan E; et al. (2007). "Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience". Journal of Neuro-Oncology. 83 (1): 61–70. doi:10.1007/s11060-006-9303-1.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid12672279" was defined multiple times with different content (see the help page). - ^ Stevens GHJ (2006). "Antiepileptic therapy in patients with central nervous system malignancies". Current Neurology and Neuroscience Reports. 6: 311–318. doi:10.1007/s11910-006-0024-9. PMID 16822352.
- ^ Lacroix M, Abi-Said D, Fourney DR; et al. (2001). "A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival". J Neurosurg. 95 (2): 190–8. PMID 11780887.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Stummer W, Pichimeier U, Meinel T; et al. (2006). "Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial". The Lancet Oncology. 7 (5): 392–401. doi:10.1016/S1470-2045(06)70665-9.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Showalter T, Andrel J, Andrews D; et al. (2007). "Multifocal Glioblastoma Multiforme: Prognostic Factors and Patterns of Progression". International Journal of Radiation OncologyBiologyPhysics,. 69 (3): 820–824. doi:10.1016/j.ijrobp.2007.03.045.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Fulton DS, Urtasun RC, Scott-Brown, I; et al. (1992). "Increasing radiation dose intensity using hyperfractionation in patients with malignant glioma. Final report of a prospective phase I-II dose response study". Journal of Neuro-Oncolog. 14 (1): 63–72. PMID 1335044.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Stupp R, Mason WP, van den Bent MJ; et al. (2005). "Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma". NEJM. 352 (10): 987–996. doi:10.1056/NEJMoa043330. PMID 15758009.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Mason WP, Mirimanoff RO, Stupp R; et al. (2006). "Radiotherapy with Concurrent and Adjuvant Temozolomide: A New Standard of Care for Glioblastoma Multiforme". Progress in Neurotherapeutics and Neuropsychopharmacology. 1: 37–52. doi:10.1017/S1748232105000054.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Temozolomide Plus Radiation Helps Brain Cancer - National Cancer Institute". Retrieved 2007-09-15.
- ^ Hoffmann D, Wildner O (2007). "Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment". Cancer Gene Ther. 14 (7): 627–39. doi:10.1038/sj.cgt.7701055. PMID 17479104.
- ^
Bonnet S, Archer S, Allalunis-Turner J; et al. (2007). "A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth". Cancer Cell. 11 (1): 37–51. doi:10.1016/j.ccr.2006.10.020.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Ford, JM, Seiferheld MS, Alger, JR; et al. (2007). "Results of the Phase I Dose-Escalating Study of Motexafin Gadolinium With Standard Radiotherapy in Patients With Glioblastoma Multiforme". International Journal of Radiation Oncology*Biology*Physics. 69 (3): 831–838. doi:10.1016/j.ijrobp.2007.04.017. PMID 17560737.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sotelo J, Briceño E, López-González MA (2006). "Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial". Ann. Intern. Med. 144 (5): 337–43. PMID 16520474.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Toler SM, Noe D, Sharma A (2006). "Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme". Neurosurgical focus. 21 (6): E10. PMID 17341043.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Briceño E, Calderon A, Sotelo J (2007). "Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme". Surgical neurology. 67 (4): 388–91. doi:10.1016/j.surneu.2006.08.080. PMID 17350410.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Maier-Hauff K, Rothe R, Scholz R; et al. (2007). "Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme". J Neurooncol. 81 (1). PMID 16773216.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Jordan; et al. (2006). "The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma". J Neurooncol. 78 (1). PMID 16314937.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "TM601-001 – Phase I/II single-dose dose open label study in patients with recurrent glioma". Transmolecular, Inc. Retrieved 2008-02-04.
- ^
Reardon D, Akabani G, Friedman A; et al. "Outcome of Newly Diagnosed Patients with High-Grade Glioma Treated with Iodine-131 Murine Anti-Tenascin Monoclonal Antibody 81C6 via Surgically Created Resection Cavities in a Phase II Trial". Proc Am Soc Clin Oncol. 20. Retrieved 2008-02-04.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^
Schlingensiepen KH, Schlingensiepen R, Steinbrecher A; et al. "Targeted tumor therapy with the TGF-beta2 antisense compound AP 12009". Cytokine Growth Factor Rev. 17 (1–7). PMID 16377233.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^
Kleinberg L, Grossman SA, Carson K; et al. (2004). "Survival of Patients With Newly Diagnosed Glioblastoma Multiforme Treated With RSR13 and Radiotherapy: Results of a Phase II New Approaches to Brain Tumor Therapy CNS Consortium Safety and Efficacy Study". Journal of Clinical Oncology. 20 (14). doi:10.1215/S1152851703000127.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^
Vredenburgh JJ, Desjardins A, Herndon JE; et al. (2007). "Bevacizumab Plus Irinotecan in Recurrent Glioblastoma Multiforme". Journal of Clinical Oncology. 25 (30). doi:10.1200/JCO.2007.12.2440.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Stylli SS, Kaye AH, MacGregor L, Howes M, Rajendra P (2005). "Photodynamic therapy of high grade glioma - long term survival". Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 12 (4): 389–98. doi:10.1016/j.jocn.2005.01.006. PMID 15925768.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eljamel MS, Goodman C, Moseley H (2007). "ALA and Photofrin(R) Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial". Lasers Med Sci. 10. doi:10.1007/s10103-007-0494-2. PMID 17926079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nieder C, Adama M, Mollsa M and Grosu AL (2006). "Therapeutic options for recurrent high-grade glioma in adult patients: Recent advances". Critical Reviews in Oncology/Hematology. 60 (3): 181–193. doi:10.1016/j.critrevonc.2006.06.007.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Krex D, Klink B, Hartmann C; et al. (2007). "Long-term survival with glioblastoma multiforme". Brain. 130 (10): 2596–2606. doi:10.1093/brain/awm204.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Williams, Ben (2006-8-28). "Brain Tumor Survivor Stories". Virtual Trials. Retrieved 2008-02-04.
{{cite web}}: Check date values in:|date=(help) - ^ Martinez R,Schackert G, Yaya-Tur R; et al. (2007). "Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme". Journal of Neuro-Oncology. 83 (1): 91–93. doi:10.1007/s11060-006-9292-0.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
Additional reading[edit]
External links[edit]
- National Cancer Institute Clinical Trials
- North America Brain Tumor Coalition
- German Brain Tumor Association
- Glioblastoma multiforme - A new Viro-therapy developed at the Hebrew University - An IsraCast article
- Emedicine.com article on glioblastoma multiforme
- Young Adults Surviving Glioblastoma support group
- Glioblastoma Images MedPix Medical Image Database
