User:Hilary Lynch/Library Sandbox/sandbox assignment
Neural tissue engineering is the use of artificial mechanisms to regenerate or replace central nervous system (CNS) or peripheral nervous system (PNS) tissues. The need for neural tissue engineering arises from the difficulty of the nerve cells and neural tissues regenerate on their own after neural damage. The PNS has some, but limited, regeneration of neural cells. Though adult stem cell neurogenesis in the CNS was generally thought to not occur, but it has been found to occur in the the hippocampus, the subventricular zone (SVZ), and spinal cord, nerve regeneration. [1] These CNS injuries can be caused by stroke, neurodegenerative disorders, trauma, or encephalopathy. A few methods currently being investigated to treat CNS injuries are: implanting stem cells directly into the injury site, delivering morphogens to the injury site, or growing neural tissue in vitro with neural stem or progenitor cells in a 3D scaffold. [2] For the PNS, a severed nerve can be reconnected and reinnervated via grafts or guidance of the existing nerve through a channel. [3]
Recent research into creating miniature cortexes, known as corticopoiesis, and brain models, known as cerebral organoids, are exciting potential techniques the field of neural tissue regeneration. The native cortical progenitors in corticopoiesis are neural tissues that could be effectively embedded into the brain.[4] Cerebral organoids are 3D human pluripotent stem cells developed into sections of the brain cortex, which show that there is a potential to isolate and develop certain neural tissues using neural progenitors. [5] Research into the innervation of PNS neurons in patients with paralysis and prosthetics may add to the knowledge of reinnervation of neural tissue in the PNS and the CNS. [6]This research is capable of making one difficult aspect of neural tissue engineering, the functional innervation of neural tissue, more manageable.
CNS[edit]
Causes of CNS Injury[edit]
There are three main causes of CNS injury: stroke, traumatic brain injury (TBI), or developmental complications. Strokes are classified as either hemorrhagic stroke (when a vessel is damaged to the point of bleeding into the brain) or ischemic (when a clot blocks the blood flow through the vessel in the brain). When a hemorrhage occurs, blood seeps into the surrounding tissue, resulting in tissue death, while ischemic hemorrhages result in a lack of blood flow to certain tissues. Traumatic brain injury is caused by external forces impacting the cranium or the spinal cord. Problems with CNS development results in abnormal tissue growth during development, thus decreasing the function of the CNS. [2]
Current Treatments and Research[edit]
Implantation of stem cells to the injury site[edit]
One method of neural tissue engineering to treat CNS injury involves culturing stem cells "in vitro" and implanting the non-directed stem cells into the brain injury site. Implanting stem cells directly into the injury site prevents glial scar formation and promotes endogenic neurogenesis, but this method runs the risk of tumors development, inflammation, and migration of the stem out of the injury location. Tumorigenesis can occur due to the uncontrolled nature of the stem cell differentiation, inflammation can occur due to rejection of the implanted cells by the host cells, and the highly migratory nature of stem cells results in the cells moving away from the injury site, thus not effecting the injury site. Other concerns of neural tissue engineering include establishing safe sources of stem cells and getting reproducible results. Alternatively, these stem cells can act as carriers for other therapies, though the positive effects of using stem cells as a delivery mechanism has not been confirmed. Direct stem cell delivery has an increased beneficial effect if they are directed to be neuronal cells "in vitro." This way, the risks associated with undirected stem cells are decreased; additionally, injuries that do not have a specific boundary could be treated efficiently. [2]

Delivery of molecules to the injury site[edit]
Molecules that promote the regeneration of neural tissue, including pharmaceuticals, growth factors known as morphogens, and microRNA (miRNA)s, can also be directly introduced to the injury site of the damaged CNS tissue. Neurogenesis has been seen in animals that are treated with psychotropic drugs via inhibition of serotonin reuptake and induction of neurogenesis in the brain. When stem cells are differentiating, the cells secrete morphogens such as growth factors to promote healthy development. These morphogens help maintain homeostasis and neural signaling pathways, and can be delivered into the injury site to promote the growth of the injured tissues. Currently, morphogen delivery has minimal benefits because of the interactions the morphogens have with the injured tissue. Morphogens that are not innate in the body have a limited effect on the injured tissue due to the physical size and their limited mobility within CNS tissue. To be an effective treatment, the morphogens must be present at the injury site at a specific and constant concentration. miRNA has also been shown to affect neurogenesis by directing the differentiation of undifferentiated neural cells. [2]
Implantation of neural tissue developed in vitro[edit]
A third method for treating CNS injuries is to artificially create tissue outside of the body to implant into the injury site. This method is potentially effective for treating injuries that consist of large cavities, where larger amounts of neural tissue needs to be replaced and regenerated. Neural tissue is grown in vitro with neural stem or progenitor cells in a 3D scaffold, forming embryoid bodies (EBs). These EBs consist of a sphere of stem cells, where the inner cells are undifferentiated neural cells and the surrounding cells are increasingly more differentiated. 3D scaffolds are used to to transplant tissue to the injury site and to make the appropriate interface between the artifical and the brain tissue. The scaffolds must be: biocompatible, biodegradable, fit injury site, similar to existing tissue in elasticity and stiffness, and support growing cells and tissues. The combination of using directed stem cells and scaffolds to support the neural cells and tissues increase the survival of the stem cells in the injury site, increasing the efficacy of the treatment. [2]
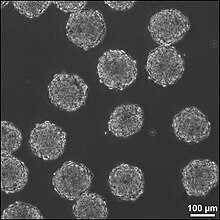
There are 6 different types of scaffolds that are being researched to use in this method for treating neural tissue injury:
- Liquid hydrogels are cross-linked hydrophobic polymer chains, and the neural stem cells are either grown on the surface of the gel or integrated into the gel during cross-linking of the polymer chains. Commonly used liquid hydrogels are Martrigel and Puramatrix. The major drawback of liquid hydrogels is there is limited protection of the cells that are transplanted.
- Supportive scaffolds are made from solid bead-shaped or microporous structures, and can act as carriers for the transplanted cells or for the growth factors that the stem cells secrete when they are differentiating. The cells adhere to the surface of the matrix in 2D layers. The supportive scaffolds are easily transplanted into the brain injury site because of the scaffold size, and provide a matrix promoting cell adhesion and aggregation, thus increasing increased healthy cell culture.
- Aligning scaffolds can be silk-based, polysaccharide-based, or other environments such as a collagen-rich hydrogel. These gels are now enhanced with micro-patterns on the surface for the promotion of neuronal outgrowths. These scaffolds are primarily used for regeneration that needs to occur in a specific orientation, such as in spinal cord injuries.
- Integrative scaffolds are mainly used to protect the transplanted cells for the mechanical forces that they are exposed to in the process of implanting them to the site of the injury. These scaffolds also decrease the likelihood of having the inflammatory cells seen at the site of the injury migrate into the scaffold with the stem cells. Blood vessels have been observed to grow through the scaffold, thus the scaffold and cells are being integrated into the host tissue.
- A combination of engineered scaffolds presents an option for a 3D scaffold that can have both the necessary patterns for cell adhesion and the flexibility to adapt to the ever changing environment of the injury site. De-cellularized ECM scaffolds is an option for scaffolds because they more closely mimc the native tissue, but these scaffolds can only currently be harvested from amputations and cadavers. [2]
These 3D scaffolds can be fabricated using particulate leaching, gas foaming, fiber bonding, solvent casting, or electrospinning techniques; each techniques creates a scaffold with different properties than the other techniques. [7]
Incorporation success of 3D scaffolds into the CNS has been shown to depend on the stage at which the cells have differentiated. Later stages provide a more efficient implantation, while earlier staged cells need to be exposed to factors that coerce the cells to differentiate and thus respond appropriately to the signals the cells will receive at the CNS injury site. [8] BDNF (brain-derived neurotrophic factor) is a potential co-factor to promote functional activation of ES cell-derived neurons into the CNS injury sites. [9]
PNS[edit]
Causes of PNS Injury[edit]
Trauma to nerves in the PNS can cause damage as severe as a severance of the nerve, splitting the nerve into a proximal and distal section. The distal nerve degenerates over time due to inactivity, while the proximal end swells over time. The distal end does not degenerate right away, and the swelling of the proximal end does not render it nonfunctional, so treatments for nerve severance by reestablishing the connection between the two ends of the nerve are being investigated. [3]
Current Treatments and Research[edit]
Surgical Reconnection[edit]
One method to treat PNS injury is surgical reconnection of the severed nerve, taking the two ends of the nerve and reconnecting them. When suturing the nerves together, the fascicles of the nerve are connected together, bridging the nerve back together. Though this method works for severances that create a small gap between the proximal and distal nerve ends, this method does not work over gaps of greater distances due to the tension that must be put on the nerve endings. This tension results in the nerve degeneration, and therefore the nerve cannot regenerate and form a functional neural connection. [3]
Tissue Grafts[edit]
Autologous tissue grafts transplant graft nerves from the patient to fill the gap between either end of the injured nerve. These nerves are typically cutaneous nerves, but other nerves have been researched as well with encouraging results. These autologous nerve grafts are the current gold standard for PNS nerve grafting because of the highly biocompatible nature of the nerve graft, but there are issues concerning harvesting the nerve from the patients themselves and having a large store of autologous grafts in mass.
Nonautologous and acellular grafts (including ECM-based materials) are tissues that do not come from the patient, but instead can be harvested from cadavers (known as allogenic tissue) or animals (known as xenogeneic tissue). While these tissues have an advantage over autologous tissue grafts because the tissue does not need to be taken from the patient, difficulty arises with the potential of disease transmission and thus immunogenic problems. Methods of eliminating the immunogenic cells, thus leaving behind only the ECM-components of the tissue, are currently being investigated to increase the efficacy of nonautologous tissue grafts. [3]
Guidance[edit]
Guidance methods of PNS regeneration use nerve guide channels to help axons regrow along the correct path, and may guide the growth factors secreted by both ends of the nerve to promote growth. Guidance methods reduce scaring of the nerves, increasing the functionality of the nerves to transmit action potentials. Two types of materials are used in guidance methods of PNS regeneration: natural-based materials and synthetic materials.
Natural-based materials are modified scaffolds stemming from ECM components and glycosaminoglycans. Laminin, collagen, and fibronectin, all ECM components, guide axonal development and promote neural stimulation and activity. Other molecules that have the potential to promote nerve repair are: hyaluronic acid, fibrinogen, fibrin gels, self-assembling peptide scaffolds, alginate, agarose, and chitosan.
Synthetic materials also provide an another method for tissue regeneration in which the graft's chemical and physical properties can be controlled. Since the properties of a material may be specified for the situation in which it is being used, synthetic materials are an attractive option for PNS regeneration. The use of synthetic materials come with certain concerns, such as: easy formation of the graft material into the necessary dimensions, biodegradable, sterilizable, tear resistant, easy to operate with, low risk of infection, and low inflammation response due to the material. The material must also maintain the channel during the nerve regeneration. Currently, a the materials most commonly researched mainly focus on polyesters, but biodegrable polyurethane, other polymers and biodegradable glass are also being investigated. Other possibilities for synthetic materials are conducting polymers and polymers biologically modified to promote cell axon growth and maintain the axon channel. [3]
Difficulty of Neural Tissue Engineering Research[edit]
Because there are so many factors that contribute to the success or failure of neural tissue engineering, there are many difficulties that arise in using neural tissue engineering to treat CNS and PNS injuries. First, the therapy needs to be delivered to the site of the injury. This means that the injury site needs to be accessed, whether by surgery or drug delivery. Both of these methods for access to the injury have inherent risks and difficulties in themselves. A second concern is the keeping the therapy at the site of the injury; stem cells have a tendency to migrate out of the injury site to other sections of the brain, thus the therapy is not as effective as it could be as when the cells stay at the injury site. Additionally, the delivery of stem cells and other morphogens to the site of injury can cause more harm if they induce tumorogenesis, inflammation, or other unforeseen effects. Finally, the findings in laboratories may not translate to practical clinical treatments, thus what may be successful in a lab, or even an animal model of the injury, may not be effective in a human patient. [10]
Related Research[edit]
Modeling Brain Tissue Development in vitro[edit]
Two models for brain tissue development are cerebral organoids and corticopoiesis. These models provide an "in vitro" model for normal brain development,[5] but they can be manipulated to represent neural defects. Therefore, the mechanisms behind healthy and malfunctioning development can be studied by researchers using these models.[5] These tissues can be made with either mouse embryonic stem cells (ESC)s or human ESCs. Mouse ESCs are cultured in a protein called Sonic Hedgehog inhibitor to promote the development of dorsal forebrain and study cortical fate. [4] This method has been shown to produce axonal layers that mimic a broad range of cortical layers. [11] Human ESC-derived tissues use pluripotent stem cells to form tissues on scaffold, forming human EBs. These human ESC-derived tissues are formed by culturing human pluripotent EBs in a spinning bioreactor.[5]
Targeted Reinnervation[edit]
Targeted reinnervation is a method to reinnervate the neural connections in the CNS and PNS, specifically in paralyzed patients and amputees using prosthetic limbs. Currently, devices are being investigated that take in and record the electrical signals that are propagated through neurons in response to a person's intent to move. This research could shed light on how to reinnervate the neural connections between severed PNS nerves and the connections between the transplanted 3D scaffolds into the CNS.[6]
References[edit]
- ^ Temple, Sally (1). "The development of neural stem cells". Nature. 414 (6859): 112–117. doi:10.1038/35102174. PMID 11689956. S2CID 4302192.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ a b c d e f Forraz, N.; Wright, KE; Jurga, M.; McGuckin, CP (2013). "Experimental therapies for repair of the central nervous system: stem cells and tissue engineering". Journal of Tissue Engineering and Regenerative Medicine. 7 (7): 523–536. doi:10.1002/term.552. PMID 22467493. S2CID 42424867.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b c d e Schmidt, Christine (4). "Neural Tissue Engineering: Strategies for Repair and Regeneration". Annuls Review of Biomedical Engineering. 5: 293-347. doi:10.1146 (inactive 2023-08-01). PMID 14527315.
{{cite journal}}: Check|doi=value (help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: DOI inactive as of August 2023 (link) - ^ a b Gaspard, N. (15). "Making cortex in a dish: In vitro corticopoiesis from embryonic stem cells". Cell Cycle. 8 (16): 2491–6. doi:10.4161/cc.8.16.9276. PMID 19597331. S2CID 6837572.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d Lancaster, Madeline A.; Renner, Magdalena; Martin, Carol-Anne; Wenzel, Daniel; Bicknell, Louise S.; Hurles, Matthew E.; Homfray, Tessa; Penninger, Josef M.; Jackson, Andrew P.; Knoblich, Juergen A. (28). "Cerebral organoids model human brain development and microcephaly". Nature. 501 (7467): 373–379. doi:10.1038/nature12517. PMC 3817409. PMID 23995685.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ a b Tenore, Francesco (2011). "Revolutionizing Prothetics: Devices for Neural Integration". Johns Hopkins APL Technical Digest. 30 (3): 230–39.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ko, Junghyuk; Mohtaram, Nima Khadem; Ahmed, Farid; Montgomery, Amy; Carlson, Michael; Lee, Patrick C.D.; Willerth, Stephanie M.; Jun, Martin B.G. (2). "Fabrication of poly (ϵ-caprolactone) microfiber scaffolds with varying topography and mechanical properties for stem cell-based tissue engineering applications". Journal of Biomaterials Science, Polymer Edition. 25 (1): 1–17. doi:10.1080/09205063.2013.830913. hdl:1828/7315. PMID 23998440. S2CID 205620361.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ Sheen, Volney L.; Arnold, Matthew W.; Wang, Youzhen; MacKlis, Jeffrey D. (1999). "Neural precursor differentiation following transplantation into neocortex is dependent on intrinsic developmental state and receptor competence". Experimental Neurology. 158 (1): 47–62. doi:10.1006/exnr.1999.7104. PMID 10448417. S2CID 12291422.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Copi, Andrea; Jüngling, Kay; Gottmann, Kurt (19). "Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network". Journal of Neurophysiology. 94 (6): 4538–4543. doi:10.1152/jn.00155.2005. PMID 16293594.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ LaPlaca, Michele. "Personal Interview".
- ^ Gaspard, Nicolas; Bouschet, Tristan; Hourez, Raphael; Dimidschstein, Jordane; Naeije, Gilles; Van Den Ameele, Jelle; Espuny-Camacho, Ira; Herpoel, Adèle; Passante, Lara; Schiffmann, Serge N.; Gaillard, Afsaneh; Vanderhaeghen, Pierre (18). "An intrinsic mechanism of corticogenesis from embryonic stem cells" (PDF). Nature. 455 (7211): 351–357. doi:10.1038/nature07287. PMID 18716623. S2CID 2611446.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help)
