User:KYPark/sandbox
Hypercholesterolemia[edit]
High-density lipoprotein (HDL) is one of the five major groups of lipoproteins, which, in order of molecular size, largest to smallest, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and HDL. Lipoprotein molecules enable the transportation of lipids (fats), such as cholesterol, phospholipids, and triglycerides, within the water around cells (extracellular fluid), including the bloodstream.
고밀도 지질단백(HDL)은 분자 크기 순으로 킬로미크론, 극저밀도 지질단백 (VLDL), 중밀도 지질단백 (IDL), 저밀도 지질단백 (LDL), 고밀도 지질단백 (HDL) 등 다섯 지질단백질의 하나다.
Because of the high cost of directly measuring HDL and LDL protein particles, blood tests are commonly performed for the surrogate value, HDL-C, i.e. the cholesterol associated with ApoA-1/HDL particles. In healthy individuals, about 30% of blood cholesterol, along with other fats, is carried by HDL.[1] This is often contrasted with the amount of cholesterol estimated to be carried within low-density lipoprotein particles, LDL, and called LDL-C. HDL particles remove fats and cholesterol from cells, including within artery wall atheroma, and transport it back to the liver for excretion or re-utilization; thus the cholesterol carried within HDL particles (HDL-C) is sometimes called "good cholesterol" (despite being the same as cholesterol in LDL particles). Those with higher levels of HDL-C tend to have fewer problems with cardiovascular diseases, while those with low HDL-C cholesterol levels (especially less than 40 mg/dL or about 1 mmol/L) have increased rates for heart disease.[2] Higher native HDL levels are correlated with better cardiovascular health;[3] however, it does not appear that further increasing one's HDL improves cardiovascular outcomes.[4]
Structure and function[edit]
HDL is the smallest of the lipoprotein particles. It is the densest because it contains the highest proportion of protein to lipids. Its most abundant apolipoproteins are apo A-I and apo A-II.[5] The liver synthesizes these lipoproteins as complexes of apolipoproteins and phospholipid, which resemble cholesterol-free flattened spherical lipoprotein particles; the complexes are capable of picking up cholesterol, carried internally, from cells by interaction with the ATP-binding cassette transporter A1 (ABCA1). A plasma enzyme called lecithin-cholesterol acyltransferase (LCAT) converts the free cholesterol into cholesteryl ester (a more hydrophobic form of cholesterol), which is then sequestered into the core of the lipoprotein particle, eventually causing the newly synthesized HDL to assume a spherical shape. HDL particles increase in size as they circulate through the bloodstream and incorporate more cholesterol and phospholipid molecules from cells and other lipoproteins, for example by the interaction with the ABCG1 transporter and the phospholipid transport protein (PLTP).
HDL transports cholesterol mostly to the liver or steroidogenic organs such as adrenals, ovary, and testes by both direct and indirect pathways. HDL is removed by HDL receptors such as scavenger receptor BI (SR-BI), which mediate the selective uptake of cholesterol from HDL. In humans, probably the most relevant pathway is the indirect one, which is mediated by cholesteryl ester transfer protein (CETP). This protein exchanges triglycerides of VLDL against cholesteryl esters of HDL. As the result, VLDLs are processed to LDL, which are removed from the circulation by the LDL receptor pathway. The triglycerides are not stable in HDL, but are degraded by hepatic lipase so that, finally, small HDL particles are left, which restart the uptake of cholesterol from cells.
The cholesterol delivered to the liver is excreted into the bile and, hence, intestine either directly or indirectly after conversion into bile acids. Delivery of HDL cholesterol to adrenals, ovaries, and testes is important for the synthesis of steroid hormones.
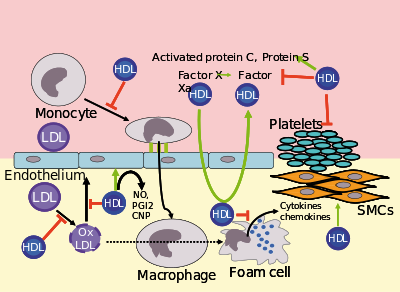
Several steps in the metabolism of HDL can participate in the transport of cholesterol from lipid-laden macrophages of atherosclerotic arteries, termed foam cells, to the liver for secretion into the bile. This pathway has been termed reverse cholesterol transport and is considered as the classical protective function of HDL toward atherosclerosis.
However, HDL carries many lipid and protein species, several of which have very low concentrations but are biologically very active. For example, HDL and its protein and lipid constituents help to inhibit oxidation, inflammation, activation of the endothelium, coagulation, and platelet aggregation. All these properties may contribute to the ability of HDL to protect from atherosclerosis, and it is not yet known which are the most important. In addition, a small subfraction of HDL lends protection against the protozoan parasite Trypanosoma brucei brucei. This HDL subfraction, termed trypanosome lytic factor (TLF), contains specialized proteins that, while very active, are unique to the TLF molecule.[6]
In the stress response, serum amyloid A, which is one of the acute-phase proteins and an apolipoprotein, is under the stimulation of cytokines (IL-1, IL-6), and cortisol produced in the adrenal cortex and carried to the damaged tissue incorporated into HDL particles. At the inflammation site, it attracts and activates leukocytes. In chronic inflammations, its deposition in the tissues manifests itself as amyloidosis.
It has been postulated that the concentration of large HDL particles more accurately reflects protective action, as opposed to the concentration of total HDL particles.[7] This ratio of large HDL to total HDL particles varies widely and is measured only by more sophisticated lipoprotein assays using either electrophoresis (the original method developed in the 1970s) or newer NMR spectroscopy methods (See also: NMR and spectroscopy), developed in the 1990s.
Subfractions[edit]
Five subfractions of HDL have been identified. From largest (and most effective in cholesterol removal) to smallest (and least effective), the types are 2a, 2b, 3a, 3b, and 3c.[8] Major Lipids in body The lipids are a heterogeneous group of compounds which are relatively insoluble in water and soluble in non polar solvents. TC, TG, and phospholipids are the major lipids in the body. They are transpoted as complexes of lipid and proteins known as lipoproteins. TGs: TGs are formed by combining glycerol with three molecules of fatty acid. TGs, as major components of VLDL and chylomicrons, play an important role in metabolism.When the body requers fatty acids as an energy source, the hormone glucagon signals the breakdown of the TGs by lipase to release free fatty acids (FFA). TGs are water insoluble, non-polar neutral fats. These are not the structural components of biological membranes.TGs synthesis and storage mostly occurs in liver and adipose tissue. FFA and glycerol must be activated prior to the synthesis of TGs into acylCoA glycerol -3-phosphate respectively. Cholesterol: The name cholesterol originates from the Greek chole- (bile) andstereos (solid), and the chemical suffix-ol for an alcohol. It is an essential structural component of cell membrane, where it is required to establish proper membrane permeability and fluidity. In addition, cholesterol is an important component for the manufacture of bile acids, steroid hormones, and vitamin D. Although cholesterol is an important and necessary molecule, a high level of serum cholesterol is an indicator for diseases such as heart disease. About 20-25% of total daily cholesterol production occurs in the liver. Phospholipids: Phospholipids are TGs that are covalently bonded to aphosphate group by an ester linkage. Phospholipids perform important functions including regulating membrane permeability and in maintaining electron transport chain in mitochondria. They participate in the reverse cholesterol transport and thus help in the removal of cholesterol from the body. They are involved in signal transmission across membranes and they act as detergents and help in solubilization of cholesterol. Lipoprotein: These contists of a central core of a hydrophobic lipid (including TGs and cholesteryl estsrs) encased in a hydrophilic coat of polar phospholipid, free cholesterol and apolipoprotein. There are four main goals of lipoprotein, differing in the relative proportion of the core lipids and in the type of apoprotein. • Chylomicrons • VLDL-C particiles • IDL-C particles • LDL-C particles • HDL-C particle • Lipoprotein (a) [LP(a)]
Epidemiology[edit]
Men tend to have noticeably lower HDL levels, with smaller size and lower cholesterol content, than women. Men also have an increased incidence of atherosclerotic heart disease. Alcohol consumption tends to raise HDL levels,[9] and moderate alcohol consumption is associated with lower cardiovascular and all-cause mortality. Recent studies confirm the fact that HDL has a buffering role in balancing the effects of the hypercoagulable state in type 2 diabetics and decreases the high risk of cardiovascular complications in these patients. Also, the results obtained in this study revealed that there was a significant negative correlation between HDL and activated partial thromboplastin time (APTT).[10]
Epidemiological studies have shown that high concentrations of HDL (over 60 mg/dL) have protective value against cardiovascular diseases such as ischemic stroke and myocardial infarction. Low concentrations of HDL (below 40 mg/dL for men, below 50 mg/dL for women) increase the risk for atherosclerotic diseases.
Data from the landmark Framingham Heart Study showed that, for a given level of LDL, the risk of heart disease increases 10-fold as the HDL varies from high to low. On the converse, however, for a fixed level of HDL, the risk increases 3-fold as LDL varies from low to high.[11][12]
Even people with very low LDL levels are exposed to increased risk if their HDL levels are not high enough.[13]
Estimating HDL via associated cholesterol[edit]
Clinical laboratories formerly measured HDL cholesterol by separating other lipoprotein fractions using either ultracentrifugation or chemical precipitation with divalent ions such as Mg2+, then coupling the products of a cholesterol oxidase reaction to an indicator reaction. The reference method still uses a combination of these techniques.[14] Most laboratories now use automated homogeneous analytical methods in which lipoproteins containing apo B are blocked using antibodies to apo B, then a colorimetric enzyme reaction measures cholesterol in the non-blocked HDL particles.[15] HPLC can also be used.[16] Subfractions (HDL-2C, HDL-3C) can be measured[17] and have clinical significance. The measurement of apo-A reactive capacity can be used to measure HDL cholesterol but is thought to be less accurate.
Recommended ranges[edit]
The American Heart Association, NIH and NCEP provides a set of guidelines for fasting HDL levels and risk for heart disease.[18][19][20]
| Level mg/dL | Level mmol/L | Interpretation |
|---|---|---|
| <40 for men, <50 for women | <1.03 | Low HDL cholesterol, heightened risk for heart disease |
| 40–59 | 1.03–1.55 | Medium HDL level |
| >60 | >1.55 | High HDL level, optimal condition considered protective against heart disease |
High LDL with low HDL level is an additional risk factor for cardiovascular disease.[21]
Measuring HDL concentration and sizes[edit]
As technology has reduced costs and clinical trials have continued to demonstrate the importance of HDL, methods for directly measuring HDL concentrations and size (which indicates function) at lower costs have become more widely available and increasingly regarded as important for assessing individual risk for progressive arterial disease and treatment methods.
Electrophoresis measurements[edit]
Since the HDL particles have a net negative charge and vary by size, electrophoresis measurements have been utilized since the 1960s to both indicate the number of HDL particles and additionally sort them by size. Larger HDL particles are carrying more cholesterol.
NMR measurements[edit]
The newest methodology for measuring HDL particles, available clinically since the late 1990s[22] uses nuclear magnetic resonance fingerprinting of the particles to measure both concentration and sizes. This methodology was pioneered by researcher Jim Otvos and the North Carolina State University academic research spinoff company[23] and dramatically reduced the cost of HDL measurements.
Optimal total and large HDL concentrations[edit]
The HDL particle concentrations are typically categorized by event rate percentiles based on the people participating and being tracked in the MESA[24] trial, a medical research study sponsored by the United States National Heart, Lung, and Blood Institute.
| MESA Percentile | Total HDL particles μmol/L | Interpretation |
|---|---|---|
| >75% | >34.9 | Those with highest (Optimal) total HDL particle concentrations & lowest rates of cardiovascular disease events |
| 50–75% | 30.5–34.5 | Those with moderately high total HDL particle concentrations & moderate rates of cardiovascular disease events |
| 25–50% | 26.7–30.5 | Those with lower total HDL particle concentrations & Borderline-High rates of cardiovascular disease |
| 0–25% | <26.7 | Those with lowest total HDL particle concentrations & Highest rates of cardiovascular disease events |
| MESA Percentile | Large HDL particles μmol/L | Interpretation |
|---|---|---|
| >75% | >7.3 | Those with highest (Optimal) Large HDL particle concentrations & lowest rates of cardiovascular disease events |
| 50–75% | 4.8–7.3 | Those with moderately high Large HDL particle concentrations & moderate rates of cardiovascular disease events |
| 25–50% | 3.1–4.8 | Those with lower Large HDL particle concentrations & Borderline-High rates of cardiovascular disease |
| 0–25% | <3.1 | Those with lowest Large HDL particle concentrations & Highest rates of cardiovascular disease events |
The lowest incidence of atherosclerotic events over time occurs within those with both the highest concentrations of total HDL particles (the top quarter, >75%)[clarification needed] and the highest concentrations of large HDL particles. Multiple additional measures, including LDL particle concentrations, small LDL particle concentrations, VLDL concentrations, estimations of insulin resistance and standard cholesterol lipid measurements (for comparison of the plasma data with the estimation methods discussed above) are routinely provided in clinical testing.
Memory[edit]
Fasting serum lipids have been associated with short term verbal memory. In a large sample of middle aged adults, low HDL cholesterol was associated with poor memory and decreasing levels over a five year follow-up period were associated with decline in memory.[25]
Increasing HDL levels[edit]
Diet and lifestyle[edit]
Certain changes in lifestyle may have a positive impact on raising HDL levels:[26]
- Decreased intake of simple carbohydrates.[27][28][29][30]
- Weight loss[31]
- niacin (vitamin B3, aka nicotinic acid) supplementation[31]
- Aerobic exercise[32]
- Smoking cessation[31]
- Mild to moderate alcohol intake[33][34][35][36][37][38]
- Addition of soluble fiber to diet[39]
- Consumption of omega-3 fatty acids such as fish oil[40] or flax oil[41]
- Increased intake of cis-unsaturated fats[42]
- Consumption of medium-chain triglycerides (MCTs) such as caproic acid, caprylic acid, capric acid, and lauric acid.
- Removal of trans fatty acids from the diet[43]
Consumption of cannabis (or marijuana) has been speculated to have a positive impact on the HDL-C level. However, a study performed in 4635 patients demonstrated no effect on the HDL-C levels (P=0.78) [the mean (standard error) HDL-C values in control subjects (never used), past users and current users were 53.4 (0.4), 53.9 (0.6) and 53.9 (0.7) mg/dL, respectively].[44]
Most saturated fats increase HDL cholesterol to varying degrees and also raise total and LDL cholesterol.[45] A high-fat, adequate-protein, low-carbohydrate ketogenic diet may have similar response to taking niacin (vitamin B3) as described below (lowered LDL and increased HDL) through beta-hydroxybutyrate coupling the Niacin receptor 1.[46]
Drugs[edit]
While higher HDL levels are correlated with cardiovascular health, no increase in HDL has been proven to improve health. In other words, while high HDL levels might correlate with better cardiovascular health, specifically increasing one's HDL might not increase cardiovascular health.[4] Pharmacological therapy to increase the level of HDL cholesterol includes use of fibrates and niacin. Fibrates have not been proven to have an effect on overall deaths from all causes, despite their effects on lipids.[47]
Niacin (vitamin B3) increases HDL by selectively inhibiting hepatic diacylglycerol acyltransferase 2, reducing triglyceride synthesis and VLDL secretion through a receptor HM74[48] otherwise known as niacin receptor 2 and HM74A / GPR109A,[46] niacin receptor 1.
Pharmacologic (1- to 3-gram/day) niacin doses increase HDL levels by 10–30%,[49] making it the most powerful agent to increase HDL-cholesterol.[50][51] A randomized clinical trial demonstrated that treatment with niacin can significantly reduce atherosclerosis progression and cardiovascular events.[52] However, niacin products sold as "no-flush", i.e. not having side-effects such as "niacin flush", do not contain free nicotinic acid and are therefore ineffective at raising HDL, while products sold as "sustained-release" may contain free nicotinic acid, but "some brands are hepatotoxic"; therefore the recommended form of niacin for raising HDL is the cheapest, immediate-release preparation.[53] Both fibrates and niacin increase artery toxic homocysteine, an effect that can be counteracted by also consuming a multivitamin with relatively high amounts of the B-vitamins[citation needed], however multiple European trials of the most popular B-vitamin cocktails, trial showing 30% average reduction in homocysteine, while not showing problems have also not shown any benefit in reducing cardiovascular event rates. A 2011 niacin study was halted early because patients adding niacin to their statin treatment showed no increase in heart health, but did experience an increase in the risk of stroke.[54]
In contrast, while the use of statins is effective against high levels of LDL cholesterol, most have little or no effect in raising HDL cholesterol.[50] However, several statins - rosuvastatin and pitavastatin - have been demonstrated to significantly raise HDL levels. As statins are associated with side effects like myopathy which causes sore muscles, patients who experience these side effects may need to be given a lower dose of statin to control cholesterol.[55]
Cannabis In unadjusted analyses, past and current marijuana use were associated with lower levels of fasting insulin, glucose, HOMA-IR, BMI, and hemoglobin A1c but either current or past marijuana use was not associated with higher HDL-C levels.[44]
Lovaza has been shown to increase HDL-C.[56] However, the best evidence to date suggests it has no benefit for primary or secondary prevention of cardiovascular disease.[57]
Magnesium supplements raise HDL-C.[58]
Apo-A1 Milano, the most effective proven HDL agent, is in commercial production by a Canadian company, Sembiosys, but as of 2010 may still be several years away from clinical availability.[citation needed]
관련사항[edit]
참고자료[edit]
- 각주
- ^ "LDL and HDL Cholesterol: What's Bad and What's Good?". American Heart Association. 2 July 2009. Retrieved 8 October 2009.
- ^ Toth, Peter (2005). "The "Good Cholesterol" High-Density Lipoprotein". Circulation. 111 (5): e89–e91. doi:10.1161/01.CIR.0000154555.07002.CA. PMID 15699268. Retrieved 2 June 2011.
- ^ Sirtori, Cesare R. (October 2006). "HDL and the progression of atherosclerosis: new insights". European Heart Journal Supplements.
- ^ a b "NIH stops clinical trial on combination cholesterol treatment". National Institute of Health. National Heart, Lung, and Blood Institute (NHLBI). Retrieved 2 June 2011.
- ^ Després, Jean-Pierre. "The Atherogenic Triad of New Metabolic Risk Factors: Importance of Waist and Fasting Triglycerides as Screening Tools". Visceral Adipose Tissue and Cardiometabolic Risk: Does It Really Matter? Part 2. Retrieved 8 October 2009.[self-published source?]
- ^ http://www.ncbi.nlm.nih.gov/pubmed/23059119
- ^ Kwiterovich Jr, P. O. (2000). "The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review". The American journal of cardiology. 86 (12A): 5L–10L. doi:10.1016/S0002-9149(00)01461-2. PMID 11374859.
- ^ HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men.
- ^ Ruidavets JB, Ducimetière P, Arveiler D; et al. (January 2002). "Types of alcoholic beverages and blood lipids in a French population". J Epidemiol Community Health. 56 (1): 24–8. doi:10.1136/jech.56.1.24. PMC 1732002. PMID 11801616.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Mard-Soltani M, Dayer MR, Shamshirgar-Zadeh A, Ali-Bahar H, Nasirbagheban Z (April 2012). "The Buffering Role of HDL in Balancing the Effects of Hypercoagulable State in Type 2 Diabetes". J Applied sciences. 12 (8): 745–52. doi:10.3923/jas.2012.745.752.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rahilly, Catherine; Spiro, Avron; Vokonas, Pantel; Gaziano, J. Michael (2011). "Relation between high-density lipoprotein cholesterol and survival to age 85 years in men (from the VA normative aging study)". Am J Cardiol. 107 (8): 1173–7. doi:10.1016/j.amjcard.2010.12.015. PMID 21296318.
- ^ HB, HB (2002). "Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT)". Arch. Intern. Med. 162 (22): 2597–604. PMID 12456232.
- ^ Barter, Philip; Gotto, Antonio M.; LaRosa, John C.; Maroni, Jaman; Szarek, Michael; Grundy, Scott M.; Kastelein, John J. P.; Bittner, Vera; et al. (2007). "HDL Cholesterol, Very Low Levels of LDL Cholesterol, and Cardiovascular Events". New England Journal of Medicine. 357 (13): 1301–10. doi:10.1056/NEJMoa064278. PMID 17898099.
- ^ "National Reference System for Cholesterol - Cholesterol Reference Method Laboratory Network - HDL Cholesterol Certification Protocol for Manufacturers" (PDF). CDC. Retrieved 10 November 2013.
- ^ Warnick, G Russell; Nauck M; Rifai N (September 2001). "Evolution of Methods for Measurement of HDL-Cholesterol: From Ultracentrifugation to Homogeneous Assays". Clinical Chemistry. 47 (9): 1579–1596. PMID 11514391. Retrieved 10 November 2013.
- ^ Okazaki, Mitsuyo; Sasamoto, Keiko; Muramatsu, Toshio; Hosaki, Seijin (1997). "Evaluation of precipitation and direct methods for HDL-cholesterol assay by HPLC". Clinical Chemistry. 43 (10): 1885–90. PMID 9342008.
- ^ Hirano, Tsutomu; Nohtomi, Kyoko; Koba, Shinji; Muroi, Ayako; Ito, Yasuki (2008). "A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay". The Journal of Lipid Research. 49 (5): 1130–6. doi:10.1194/jlr.D700027-JLR200. PMID 18223297.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Cholesterol Levels". American Heart Association. Retrieved 14 November 2009.
- ^ "What Do My Cholesterol Levels Mean?" (PDF). American Heart Association. September 2007. Retrieved 14 November 2009.
- ^ "Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary" (PDF). National Heart, Lung, and Blood Institute (NHLBI). National Institutes of Health. May 2001.
- ^ ASHWOOD, E. R., BURTIS, C. A., & BRUNS, D. E. (2008). Tietz fundamentals of clinical chemistry. (6th ed., p. 415). St. Louis, MO: Saunders Elsevier.
- ^ liposcience.com
- ^ liposcience.com
- ^ mesa-nhlbi.org
- ^ Singh-manoux, A; Gimeno, D; Kivimaki, M; Brunner, E; Marmot, MG (2008). "Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study". Arteriosclerosis, thrombosis, and vascular biology. 28 (8): 1556–62. doi:10.1161/ATVBAHA.108.163998. PMC 2581752. PMID 18591462.
- ^ Fogoros, Richard N. (15 September 2009). "Raising Your HDL Levels Increasing the GOOD cholesterol". About.com. Retrieved 8 October 2009.
- ^ http://www.ajcn.org/content/77/5/1146.full
- ^ Yunsheng, Ma; Youfu, Li (April 2006). "Association between Carbohydrate Intake and Serum Lipids". Journal of the American College of Nutrition. 25 (2): 155–163. PMC 1479303. PMID 16582033. Retrieved 1 September 2012.
- ^ Siri-Tarino, Patty W; Qi Sun; Frank B Hu; Ronald Krauss (January 2010). "Saturated fat, carbohydrate, and cardiovascular disease". The American Journal of Clinical Nutrition. Retrieved 1 September 2012.
- ^ Krauss, Ronald; Patricia J Blanche; Robin S Rawlings; Harriett S Fernstrom; Paul T Williams (May 2006). "Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia". American Journal of Clinical Nutrition. 83 (5): 1025–1031. PMID 16685042. Retrieved 1 September 2012.
- ^ a b c Hausenloy DJ, Yellon DM (June 2008). "Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels". Heart. 94 (6): 706–14. doi:10.1136/hrt.2007.125401. PMID 18480348.
- ^ Spate-douglas, T; Keyser, RE (1999). "Exercise intensity: its effect on the high-density lipoprotein profile". Archives of physical medicine and rehabilitation. 80 (6): 691–5. doi:10.1016/S0003-9993(99)90174-0. PMID 10378497.
- ^ Baer, D.; Judd, J.; Clevidence, B.; Muesing, R.; Campbell, W.; Brown, E.; Taylor, P. (2002). "Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet". The American journal of clinical nutrition. 75 (3): 593–599. PMID 11864868.
- ^ Van Der Gaag, M.; Van Tol, A.; Vermunt, S.; Scheek, L.; Schaafsma, G.; Hendriks, H. (2001). "Alcohol consumption stimulates early steps in reverse cholesterol transport". Journal of lipid research. 42 (12): 2077–2083. PMID 11734581.
- ^ Hendriks, H.; Veenstra, J.; Van Tol, A.; Groener, J.; Schaafsma, G. (1998). "Moderate doses of alcoholic beverages with dinner and postprandial high density lipoprotein composition". Alcohol and alcoholism (Oxford, Oxfordshire). 33 (4): 403–410. doi:10.1093/oxfordjournals.alcalc.a008410. PMID 9719399.
- ^ Clevidence, B.; Reichman, M.; Judd, J.; Muesing, R.; Schatzkin, A.; Schaefer, E.; Li, Z.; Jenner, J.; Brown, C.; Sunkin, M.; Campbell, W. S.; Taylor, P. R. (1995). "Effects of alcohol consumption on lipoproteins of premenopausal women. A controlled diet study". Arteriosclerosis, thrombosis, and vascular biology. 15 (2): 179–184. doi:10.1161/01.ATV.15.2.179. PMID 7749823.
- ^ Cuvelier, I.; Steinmetz, J.; Mikstacki, T.; Siest, G. (1985). "Variations in total phospholipids and high-density lipoprotein phospholipids in plasma from a general population: Reference intervals and influence of xenobiotics". Clinical chemistry. 31 (5): 763–766. PMID 3987006.
- ^ Brenn, T. (1986). "The Tromsø heart study: Alcoholic beverages and coronary risk factors". Journal of epidemiology and community health. 40 (3): 249–256. doi:10.1136/jech.40.3.249. PMC 1052533. PMID 3772283.
- ^ Hermansen K, et al. "Effects of soy and other natural products on LDL:HDL ratio and other lipid parameters: a literature review.", "National Institutes of Health", 2003 Jan–Feb. Retrieved 2011 May 31.
- ^ "The Power of Fish". The Cleveland Clinic Heart and Vascular Institute. Retrieved 8 October 2009.
- ^ "Vitamins and Supplements Lifestyle Guide - Flaxseed". WebMD. Retrieved 12 August 2013.
- ^ Mensink, Ronald P.; Zock, Peter L.; Kester, Arnold D. M.; Katan, Martijn B. (2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". American Journal of Clinical Nutrition. 77 (5): 1146–55. PMID 12716665.
- ^ "Trans fat: Avoid this cholesterol double whammy". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 25 June 2010.
- ^ a b Penner, Elizabeth A.; Hannah Buettner; Murray A. Mittleman (16 May 2013). "The Impact of Marijuana Use on Glucose, Insulin, and Insulin Resistance among US Adults". American Journal of Medicine. 126 (7): 583–589. doi:10.1016/j.amjmed.2013.03.002. Retrieved 5 October 2013.
- ^ Thijssen, M.A. and R.P. Mensink. (2005). Fatty Acids and Atherosclerotic Risk. In Arnold von Eckardstein (Ed.) Atherosclerosis: Diet and Drugs. Springer. pp. 171–172. ISBN 978-3-540-22569-0.
- ^ a b Soudijn, W; Van Wijngaarden, I; Ijzerman, AP (2007). "Nicotinic acid receptor subtypes and their ligands". Medicinal Research Reviews. 27 (3): 417–33. doi:10.1002/med.20102. PMID 17238156.
- ^ Benatar, JR; Stewart, RA (2007). "Is it time to stop treating dyslipidaemia with fibrates?". The New Zealand medical journal. 120 (1261): U2706. PMID 17853928.
- ^ Meyers, CD; Kamanna, VS; Kashyap, ML (2004). "Niacin therapy in atherosclerosis". Current Opinion in Lipidology. 15 (6): 659–65. doi:10.1097/00041433-200412000-00006. PMID 15529025.
- ^ Rader, Daniel J. (2004). "Raising HDL in Clinical Practice". Raising HDL in Clinical Practice: Clinical Strategies to Elevate HDL. Retrieved 8 October 2009.
- ^ a b Brewer, H. Bryan (27 December 2005). "Raising HDL-Cholesterol and Reducing Cardiovascular Risk: An Expert Interview With H. Bryan Brewer, Jr, MD". Retrieved 8 October 2009.
- ^ Chapman, M. John; Assmann, Gerd; Fruchart, Jean-Charles; Shepherd, James; Sirtori, Cesare; European Consensus Panel on HDL-C (2004). "Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid – a position paper developed by the European Consensus Panel on HDL-C". Current medical research and opinion. 20 (8): 1253–68. doi:10.1185/030079904125004402. PMID 15324528.
- ^ Drexel, H. (2006). "Reducing risk by raising HDL-cholesterol: the evidence". European Heart Journal Supplements. 8: F23. doi:10.1093/eurheartj/sul037.
- ^ Meyers, C. Daniel; Carr, Molly C.; Park, Sang; Brunzell, John D. (2003). "Varying Cost and Free Nicotinic Acid Content in Over-the-Counter Niacin Preparations for Dyslipidemia". Annals of Internal Medicine. 139 (12): 996–1002. doi:10.7326/0003-4819-139-12-200312160-00009. PMID 14678919.
- ^ http://www.npr.org/blogs/health/2011/05/28/136678665/study-boosting-good-cholesterol-with-niacin-did-not-cut-heart-risks?ps=sh_sthdl
- ^ "When is treatment indicated for high cholesterol level?".
- ^ http://us.gsk.com/products/assets/us_lovaza.pdf
- ^ Omega-3 fatty acid#Cardiovascular disease
- ^ Rosanoff A, Seelig MS (October 2004). "Comparison of mechanism and functional effects of magnesium and statin pharmaceuticals". J Am Coll Nutr. 23 (5): 501S–505S. doi:10.1080/07315724.2004.10719389. PMID 15466951.
HMG CoA Reductase is an important enzyme in lipid and cholesterol metabolism, but it is not the only one. The statins act by inhibiting, temporarily, the enzyme, in a dose response relationship whereas the magnesium ion (Mg2+) is an important part of a complex control and regulation of this important pathway. Both lower LDL-C, some statins can raise HDL-C and lower triglycerides, but Mg supplements do both quite reliably.
External links[edit]
- Adult Treatment Panel III Full Report – National Heart, Lung, and Blood Institute
- ATP III Update 2004 – National Heart, Lung, and Blood Institute
- HDL: The good, but complex, cholesterol – Harvard Heart Letter
- HDL Cholesterol at Lab Tests Online
