User:Kim.glor/sandbox
 | This is a user sandbox of Kim.glor. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
| Kim.glor/sandbox | |
|---|---|
| |
| Scientific classification | |
| Kingdom: | |
| Division: | Murcoromycota
|
| Subdivision: | |
| Class: | Mucormycetes
|
| Order: | |
| Family: | |
| Genus: | Syncephalastrum
|
| Species: | S. racemosum
|
| Binomial name | |
| Syncephalastrum racemosum Cohn (1886)
| |
| Synonyms | |
| |
Syncephalastrum racemosum is a heterothallic filamentous fungus that is a common saprobic found abundantly in the environment, such as in soil, decaying vegetation and animal feces.[1][2] Syncephalastrum racemosum is uncommonly encountered as a human and animal pathogen, typically only in the setting of immunocompromise.[3][4] It is the only known merosporangial agent of disease in humans.[4]
History and taxonomy[edit]
The fungal species Syncephalastrum racemosum was described from Breslau, Poland in 1886 by German mycologist Ferdinand Cohn in a treatise by Joseph Schröter.[5] Syncephalastrum racemosum remains the only recognised multisporous species of the genus Syncephalastrum.[1][6] Originally, the genus Syncephalastrum was thought to be made up of a broader range of species based on the morphology of sporangiospores. The genus previously had been made up of S. nigricans, S. elegans, S. cinereum, S. fuliginosum, S. avanicum, and S. racemosum var. paucisporum.[4][7] The taxonomy within the order Mucorales was first questioned by Richard Benjamin in 1959.[7] By 1980, the taxonomy of the species was investigated and revised where the only remaining accepted species within the genus were S. racemosum and S. verruculosum.[4] Further studies investigating mating compatibility between the two species demonstrated that the two species represented variants of a single species, S. racemosum.[4] Variants of one additional, species with single-spored sporangia, S. monosporum added by Zheng and co-workers in 1988 are of uncertain taxonomic standing.[8][4] Thus, the substantial variation that exists in traits such as vesicle diameter, number of spores in the merosporangium, shape and size of the spores and color of colony of S. racemosum is not of taxonomic value.[7]
Growth and morphology[edit]

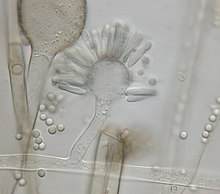
Syncephalastrum racemosum is characterized uniquely by the abundant formation of cylindrical merosporangia with deliquescent walls containing uniseriate spores.[9] Merosporangia are attached around globose vesicles called ampullae.[4][10][9] The species produces rapidly-growing colonies with aerial mycelia that can grow 0.5-1.5 cm high and 10-50 µm thick.[5][10][9] The colonies of a wooly texture are highly variable in color, where it can range from white, shades of green, olive, brown, gray, to almost black.[11][4] The hyphae are hyaline with a smooth outer membrane.[4][5] The fruiting structure morphology identifies with sporangiophores that end in ampullae. The sporangiophores grow from 30-80 µm in length and have the rod shaped merosporangia (25 × 5 µm) originating from the vesicle surface.[4][9] The vegetative mycelia of the fungus are typically identified with coenocytic or aseptate hyphae.[2][4] The cylindrical merosporangia will either develop and contain asexual sporangiospores, which give off a gray or blackish color, or dark brown zygospores will result from sexual mating.[4] Merosporangia contain about 3-18 spores, averaging at 7 spore per sack.[12] The spores do not have specific morphology and are colorless from light microscopy.[5] The colonies are commonly mistaken for other fungal species due to the resemblance in morphology. Colonies of darker color look similar to Rhizopus stolonifer, with black heads produced on branches of aerial mycelia.[10][12] S. racemosum is also often confused with members of the genus Aspergillus, especially A. niger where the only difference is the aseptate, ribbon-like mycelium and merosporangial sack in Syncephalastrum colonies.[4]
Physiology[edit]
Colonies of Syncephalastrum racemosum grow rapidly when cultured on artificial nutrient media, where sporangiophores will start to germinate within the first 6-8 hours and cover the medium within a week.[4][7] The fungal species has a growth range between 17–40 °C (63–104 °F), but it is thought that the actual range is much broader since it was later found that there was no growth below 5 °C (41 °F).[13] Sporulation of the fungus occurs readily at room temperature and temperatures above 37 °C (99 °F).[4] Growth of S. racemosum colonies are relatively easily obtained on multiple artificial media such as yeast extract agar, oatmeal agar, and potato sucrose malt extract agar.[9][14] Ideal culturing conditions were at temperatures of 36–40 °C (97–104 °F), and the cultures can be stored at 5 °C (41 °F).[9] For mating situations, ideal conditions are 25 °C (77 °F) on yeast extract agar.[9] Culturing in Czapek yeast agar(CYA) and malt extract agar(MEA) resulted in sparse gray colonies. On the other hand, growth on G25N media at 37 °C (99 °F) resulted in dense to floccose, gray colonies(20-30 mm diameter) with a pale color on the reverse.[13] S. racemosum is one of the most tolerant Mucorales to low water activity(aw), where the minimum aw required for growth is 0.84.[13]
Habitat and ecology[edit]
The distribution of S. racemosum is very broad and can be found worldwide, but it mainly originates within tropical and subtropical areas.[12] The wide-spread species has been isolated from various environmental sources and resonates mostly in sites rich with organic matter. Multiple soil sources have lead to isolates in India, southern U.S., Panama, and Israel.[4] Other sources such as outdoor air sources and dust samples in houses trace to locations of Nigeria and Britain.[4] Plants and food variations have also been areas where the fungus has been found as a contaminant, such as bird feed, oats, wheat, soya, nuts, cereal, spices, rice, sugar cane, corn, and barley.[4][13] Samples from different water sources, bird droppings, and plant compost have also resulted in colonies of S. racemosum when cultured.[4] Cellular materials of Syncephalastrum racemosum are often considered as allergenic, but the fungus is rarely found to be associated with human infectious disease.[1] When S. racemosum is inhaled or ingested from contaminated food, the specimen can be extracted from stool samples but has yet to be linked to any invasive diseases. These fungal infections are ruled as insignificant.[1][4] S. racemosum is more widely known to infect plant material than humans. The species has also been found to be an endophytic fungus in some cases, where isolates have been found from leaves of Markhamia tomentosa.[15]
Clinical significance[edit]
S. racemosum is more widely recognized as nonpathogenic to humans than to have a large clinical significance. The fungal species is within the biosafety risk group RG2, since it tends to only infect immunosuppressed individuals or after direct contact with open wounds.[16][17] Primarily, cutaneous mucormycosis by S. racemosum is involved in contamination to wound sites after exposure of the open wounds to airborne or soil-borne spores.[4] The class Mucoromycetes generally is correlated with opportunistic pathogenicity. The first recorded pulmonary infection with S. racemosum was discovered in an immunocompromised person after receiving a liver transplant.[1] There have also been a handful of subcutaneous infection cases involving isolation of the species from toe or finger nails of onychomycosis patients, especially if the patient had previous damage to the nail.[1][18][19] Other isolates have been recorded from non-human hosts. S. racemosum has been determined as a causal agent for a case of bovine mycotic abortion.[9] Also pathogenetic effects have been seen in leafcutter ants after exposure, which has lead to the further research and application of the fungus in biological control of pest management.[20]
A single case of wound infection by S. racemosum reported following penetration trauma of the abdomen by a steel rod resolved with daily intravenous dosage of 300 mg of amphotericin B over 29 days.[3] Successful treatment of invasive fungal infections by Syncephalastrum racemosum depends on early diagnosis, therapy for the symptoms, and if necessary invasive surgery to remove infected tissue.[3] Usually specific antifungal therapy is not needed. Often S. racemosum infection can be cured after resolving other immunosuppressive factors such as diabetes. In extreme cases where drug treatment is needed, antifungal agents amphotericin B, nystatin, and primaricin showed good susceptibility.[4] The species showed resistance to the drug saperconazole.[4]
Mycotoxins[edit]
Syncephalastrum racemosum has been found to produce multiple mycotoxins in vitro, where some have been proved to suppress the cell division rate in plant root cells.[4] As a result, S. racemosum is heavily studied as a plant pathogen. However, studies with other specimen have been carried out showing the effect of the mycotoxins produced by S. racemosum. When mice were intravenously exposed to three isolates of S. racemosum, it was found that two of the three isolates were mildly toxic to the specimen.[13] The injections led to death of the mice exposed to the fungal species indicating mycotoxin presence within some of the strains. No other details were recorded from the experiment. In vivo studies within rabbits showed that infection with the spores of S. racemosum failed to result in any pulmonary or cerebral invasive disease.[4] This further supports the idea of low virulence in mammals and is unlikely to be of large clinical significance in humans.
Potential usage[edit]
The species S. racemosum has shown mutualistic relationships as plant endophytes. Isolates of Syncephalastrum racemosum from M. tomentosa plant leaves have displayed antifungal and antiproliferative activities.[15] When the isolated strain was tested against pathogenic fungi in dual cultures and poisoning food assays, S. racemosum showed to be very effective in anti-proliferative and antagonistic activity against plant pathogenic fungus Fusarium oxysporum. The species has the ability to be used in plant protection methods.[15] Syncephalastrum racemosum also can potentially be used to benefit human health. Isolates of the species showed inhibition in cell growth of HeLa cancer cell line.[15] The species has high contents of chitosan within cell walls, which can be used in a variety of applications when extracted including support in enzyme immobilization.[21]
References[edit]
- ^ a b c d e f Gassner, Marika; Zainah, Hadeel; Arback, Dima; Li, Hanhan; Stone, Chad; Ramesh, Mayur; Rivers, Emanuel (2013). "A rare case of Syncephalastrum racemosum invasive pulmonary infection after transplantation". Chest. 144 (4): 242A. doi:10.1378/chest.1704244. Retrieved 13 October 2017.
- ^ a b O'Donnell, Kerry L. (1979). Zygomycetes in culture. Athens, GA: University of Georgia. ISBN 0-935460-01-2.
- ^ a b c Schlebusch, Sanmarié; Looke, David F.M. (Nov 2005). "Intraabdominal Zygomycosis Caused by Syncephalastrum racemosum Infection Successfully Treated with Partial Surgical Debridement and High-Dose Amphotericin B Lipid Complex". Journal of Clinical Microbiology. 43 (11): 5825–5827. doi:10.1128/JCM.43.11.5825-5827.2005.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y Ribes, Julie A.; Vanover-Sams, Carolyn L.; Baker, Doris J. (April 2000). "Zygomycetes in Human Disease". Clinical Microbiology Reviews. 13 (3): 236–301.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b c d Zycha, H.; Siepmann, R.; Linnemann, G. (1969). Mucorales. Lehre, Germany: J Cramer.
{{cite book}}:|access-date=requires|url=(help) - ^ "Syncephalastrum racemosum". Mycobank. International Mycological Association. Retrieved 12 October 2017.
- ^ a b c d Benjamin, R. K. (1959). "The Merosporangiferous Mucorales". Aliso: A Journal of Systematic and Evolutionary Botany. 4 (2): 321–433. doi:10.5642/aliso.19590402.05.
{{cite journal}}:|access-date=requires|url=(help) - ^ Zheng, R.Y.; Chen, G.Q.; Hu, F.M. (1988). "Monosporous varieties of Syncephalastrum". Mycosystema. 1: 35–52.
- ^ a b c d e f g h Howard, Dexter H. (2007). Pathogenic fungi in humans and animals (2nd ed.). New York, NY: Dekker. ISBN 0824706838.
- ^ a b c Kwon-Chung, K. June; Bennett, Joan E. (1992). Medical mycology. Philadelphia: Lea & Febiger. ISBN 0812114639.
- ^ St-Germain, Guy (2003). Identifying filamentous fungi (1 ed.). Belmont, CA: Star Pub. ISBN 0-89863-177-7.
- ^ a b c Onions, A.H.S.; Allsopp, D.; Eggins, H.O.W. (1981). Smith's introduction to industrial mycology (7th ed.). London, UK: Arnold. ISBN 0-7131-2811-9.
- ^ a b c d e Pitt, John I.; Hocking, Ailsa H. (2009). Fungi and Food Spoilage (3 ed.). New York, NY: Springer. pp. 165–166. ISBN 978-0-387-92206-5.
{{cite book}}:|access-date=requires|url=(help) - ^ Huang, Wen-Ku; Sun, Jian-Hua; Cui, Jiang-Kuan; Wang, Gang-Feng; Kong, Ling-An; Peng, Huan (February 4, 2014). "Efficacy evaluation of fungus Syncephalastrum racemosum and nematicide avermectin against the root-knot nematode Meloidogyne incognita on cucumber". 9 (2): e89717. doi:10.1371/journal.pone.0089717.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: unflagged free DOI (link) - ^ a b c d Ibrahim, Mutiat; Kaushik, Nutan; Sowemimo, Abimbola; Chhipa, Hemraj; Koekemoer, Trevor; van de Venter, Maryna; Odukoya, Olukemi A. (Dec 9, 2016). "Antifungal and antiproliferative activities of endophytic fungi isolated from the leaves of Markhamia tomentosa". Pharmaceutical Biology. 55 (1): 590–595. doi:10.1080/13880209.2016.1263671.
{{cite journal}}:|access-date=requires|url=(help) - ^ "CBS 112387". Westerdijk Fungal Biodiversity Institute. Westerdijk Fungal Biodiversity Institute. Retrieved 13 October 2017.
- ^ UAMH Centre for Global Microfungal Biodiversity. "UAMH 197- Syncephalastrum racemosum". UAMH Centre for Global Microfungal Biodiversity. Retrieved 23 November 2017.
- ^ Pavlovic, Milos D; Bulajic, Nina (2006). "Great toenail onychomycosis caused by Syncephalastrum racemosum". Dermatology Online Journal. 12 (1): 7. Retrieved 22 November 2017.
- ^ Jindal, N; Kalra, N; Arora, D; Bansal, R (2016). "Onychomycosis of toenails caused by Syncephalastrum racemosum: A rare non-dermatophyte mould". Indian Journal of Medical Mycology. 34 (2): 257–258. doi:10.4103/0255-0857.176844. Retrieved 13 October 2017.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Barcoto, Mariana; Pedrosa, Felipe; Bueno, Odair; Rodrigues, Andre (May 2017). "Pathogenic nature of Syncephalastrum in Atta sexdens rubropilosa fungus gardens". Pest Management Science. 73 (5): 999–1009. doi:10.1002/ps.4416. Retrieved 13 October 2017.
- ^ Amorim, R.V.S; Melo, E.S.; Carneiro-da-Cunha, M.G.; Ledingham, W.M; Campos-Takaki, G.M. (August 2003). "Chitosan from Syncephalastrum racemosum used as a film support for lipase immobilization". Bioresource Technology. 89 (1): 35–39. doi:10.1016/S0960-8524(03)00035-X.
{{cite journal}}:|access-date=requires|url=(help)
