User:Mphil7/sandbox
Remyelination is the process propagating Oligodendrocyte Precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS [1] The process creates a thinner myelin sheath than normal, but it helps to protect the axon from further damage, from overall degeneration, and proves to increase conductance once again. Demyelinating diseases, such as Multiple Sclerosis, have been of utmost interest within the last couple of decades. Recent research is uncovering some of the many unknown pathways involved with remyelination in hopes of battling demyelinating diseases like MS which can ultimately cripple a person. While no treatment exists yet in preventing remyelination failure in the chronic stages of these diseases, future research may yet prove to unlock key pathways that can be targeted.
Function[edit]
Remyelination is activated and regulated by a variety of factors surrounding lesion sites that control the migration and differentiation of Oligodendrocyte Precursor Cells. Remyelination looks different than developmental myelination in the structure of the myelin formed. Reasons for this are unclear, but proper function of the axon is restored regardless. Perhaps of most interest are the inhibition and promotion factors of this physiological process.
Characteristics of remyelinated axon[edit]
The most notable evidence that remyelination has taken place on an axon is its thin myelin sheath created by an oligodendrocyte. It is unclear why remyelination causes the thinner sheaths. This phenomenon is shown in the g-ratio, the ratio of axon circumference to myelin circumference. So then, this value will always be less than 1, but remyelinated axons tend to have values closer to 1 (greater in actual value) than those myelinated naturally. The g-ratio differences are in g-ratio are less apparent on smaller axons. [1]
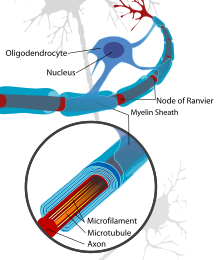
The thinner myelin not only restores protection of the axon from degredation, but also restores a faster conduction velocity. The conduction velocity, however, is not as strong as naturally myelinated axons and the Nodes of Ranvier are inclined to be wider which results in less coverage in the axon by myelin than what is natural. [2]
OPC involvement[edit]
Oligodendrocyte Precursor Cells, or OPC's, are the main cell responsible for the remyelination of demyelinated axons. There are two physiological changes that must occur to OPC's for remyelination to occur.[1] Once a signal is sent that remyelination is needed, OPC's will first migrate to damaged axon. This process may be signaled or enhanced by microglia or astrocytes at the injured axon site that stimulate migratory OPC pathways [1] From there the cells must differentiate from being progenitors to being pre-oligodendrocytes, then premyelinating oligodendrocytes, and finally mature oligodendrocytes [2]. These oligodendrocytes can then wrap damaged axons with new myelin sheaths. This process of differentiation through several phases has many involved and direct pathways and factors necessary for the completion of this process. It is easy to completely stop remyelination with the failure of a number of pathways.
Propagation factors[edit]
One of the difficulties of studying remyelination is the variety of factors that play a role in differentiating oligodendrocyte progenitors. While some factors promote and and others inhibit, still some factors that are known to be involved are yet not understood enough to know whether it promotes, inhibits, or does both. Many factors are poorly understood and subject to much change as research is done.
Cytokines and chemokines[edit]
Cytokines mediate inflammatory responses that promote pathogen and debris clearance so that further tissue damage is avoided[1][3][2] .Too much of can mean cell death but failure to propagate cytokines at all in remyelination results in a lack of debris clearance at a damaged axon site; this build up of myelin and oligodendrocyte debris has been shown to inhibit the differentiation of Oligodendrocyte Precursor Cells[1]. Specifically, cytokines promote TNFR2 and eventually TNF-alpha which plays a key role in OPC differentiation [3].
It has also be shown that chemokines are involved in guiding immune cells to sites of axon lesions to facilitate inflammation and debris clearance as well as possibly guiding OPCs migration to lesion sites. So then, chemokines are directly involved with both migration and differentiation of OPCs [3]. The specific chemokines involved with each of these two processes is known: CXCL12 is related to migration and differentiation is increased with an increase in CXCR7 and a decrease in CXCR4[3]. In certain demyelinating diseases CXCL12 has been shown to be decreased, possibly playing a role in demyelination failure. Still much is to be researched in this field, as certain chemokines like CXCR2 plays a role in inflammation and repair but in an unknown manner over much controversy[3].
Signaling Pathways[edit]
LINGO1 is one of the biggest pathways under research for trying to understand and promote remyelination. It is thought to inhibit not only axon regeneration but also regulate oligodendrocyte maturation by inhibiting OPC differentiation. It has been shown that when a LINGO1 antagonist is given in a research setting, OPC differentiation and thus remyelination is promoted at demyelinated sites. LINGO1 gene expression also is known to activate RhoA which may also play a part in inhibition[3][1][2]. Myelin debris build up might be responsible for the promotion of the LINGO1 gene and overall inhibition [2][4].
The Notch-1 receptor pathway is another pathway that inhibits the differentiation of OPCs [2]. When the ligands Jagged1 and Delta, produced by axons, neurons, and astrocytes, are stimulated and bind to the membrane, oligodendrocyte maturation is inhibited. This pathway may also be facilitating migration despite its differentiation inhibition [3]. There may be other ligands that have either promoting or inhibiting effects when attached to the Notch-1 receptor [1].
The Wnt-β-Catelin pathway has been shown to also inhibit remyelination when it is dysregulated in the body. Demyelinating diseases have been shown to cause this dysregulation. Possible genes involved inside this pathway are TCF4 and OLIG2 which are both expressed in high amounts in areas where remyelination has failed from demyelinating diseases [5] [2].
Transcription Factors[edit]
Gene expression may be the most important factor in understanding remyelination and can hold the key to understanding how to treat demyelinating diseases. OLIG1 has been shown to be critical in developmental myelination and may also be important in remyelination [3]. OLIG2 and TRF4 have also been shown to be important especially in the Wnt-β-Catelin Pathway, most likely in inhibiting remyelination. NKX2-2 is a gene coding for a protein that may increase the number of OPCs in low amounts, possibly working with OLIG2 in some way to differentiate OPCs to mature oligodendrocytes [3]. As more genes involved in remyelination are found and cross linked more will be understood about promotion and inhibition.
Other factors[edit]
It is known that as age increases, so does the efficiency (that is the speed and magnitude) of remyelination at demyelinated axons. This is probably linked with down regulation of certain expressed genes with increased age. The research of this is particularly important with the elderly whose myelin and axons are more prone to be degenerated in the CNS[6] [1].
Toll-Like Receptors are also involved in remyelination, most likely inhibiting remyelination and OPC differentiation. There are a variety of types of these receptors, but a majority of them tend to increase, especially in the chronic stages of demyelinating diseases, suggesting that they may be involved with remyelination failure[3][2].
MicroRNA is not well understood but may play a minor or major role in remyelination. MicroRNA may have a role in reduction of CD47 which promotes phagocytosis of myelin [3].
Disease treatment[edit]
Understanding completely the inhibiting and promoting factors of OPC's seams to be the key in battling demyelinating diseases such as Multiple Sclerosis that cause remyelination to fail. Not only are the inhibition factors being looked at as ways to stop remyelination failure, but promotion factors are being looked at to facilitate remyelination in the face of inhibited processes. Stem cell research is also ongoing in seeing how to differentiate neural stem cells into mature oligodendrocytes that will activate at demyelinated sites [3].
Multiple Sclerosis[edit]
Multiple Sclerosis, or MS, is the most prominent of the demyelinating diseases affecting at least 1 in 1000 people world wide on average. The ratio is much higher than that in certain areas of the world. While the early stages of multiple sclerosis are less discernable, the chronic stages can greatly reduce an individuals quality of life by limiting motor function. The demyelinating disease attacks the myelin of axons in the Central Nervous System through autoimmune defects. While remyelination is very efficient in the early stages of Multiple Sclerosis, it causes remyelination to fail in the more Chronic stages[1]. As axons are left bare, without myelin, their conduction velocity goes down due to a lack in increased potential between the Nodes of Ranvier. Not only does conduction go down, but a naked axon is also much more like to degrade completely, resulting in complete loss of function for certain motor functions.
Future research[edit]
Still much is not understood concerning remyelination. New pathways are being discovered constantly in the areas of gene regulation, antibody use as antagonists, and promotion of stem cells to differentiate. There are many regulation factors, such as Lingo-1, Olig-1, Id2, Id4, Hes5, and Sox6, that are not very well understood in their role that may hold the key to developing new treatments for demyelinating diseases[1][7]. There is much funding in the area of remyelination, name the organization "The Myelin Project" is constantly raising money and awareness about remyelination in order to treat demyelinating diseases and their devastating effects on lives.
References[edit]
- ^ a b c d e f g h i j k Franklin, Robin J. M.; Ffrench-Constant, Charles (November 2008). "Remyelination in the CNS: from biology to therapy". Nature Reviews Neuroscience. 9 (11): 839–855. doi:10.1038/nrn2480. PMID 18931697.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b c d e f g h Hanafy, Khalid A.; Sloane, Jacob A. (1 December 2011). "Regulation of remyelination in multiple sclerosis". FEBS Letters. 585 (23): 3821–3828. doi:10.1016/j.febslet.2011.03.048. PMID 21443876.
- ^ a b c d e f g h i j k l Patel, Jigisha R.; Klein, Robyn S. (1 December 2011). "Mediators of oligodendrocyte differentiation during remyelination". FEBS Letters. 585 (23): 3730–3737. doi:10.1016/j.febslet.2011.04.037. PMC 3158966. PMID 21539842.
- ^ Mi, Sha; Miller, Robert H.; Tang, Wei; Lee, Xinhua; Hu, Bing; Wu, Wutain; Zhang, Yiping; Shields, Christopher B.; Zhang, Yongjie; Miklasz, Steven; Shea, Diana; Mason, Jeff; Franklin, Robin J. M.; Ji, Benxiu; Shao, Zhaohui; Chédotal, Alain; Bernard, Frederic; Roulois, Aude; Xu, Janfeng; Jung, Vincent; Pepinsky, Blake (1 March 2009). "Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells". Annals of Neurology. 65 (3): 304–315. doi:10.1002/ana.21581. PMID 19334062.
- ^ Fancy, Stephen P.J.; Baranzini, Sergio E.; Zhao, Chao; Yuk, Dong-In; Irvine, Karen-Amanda; Kaing, Sovann; Sanai, Nader; Franklin, Robin J.M.; Rowitch, David H. (10 June 2009). "Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS". Genes & Development. 23 (13): 1571–1585. doi:10.1101/gad.1806309. PMC 2704469. PMID 19515974.
- ^ Shen, Siming; Sandoval, Juan; Swiss, Victoria A.; Li, Jiadong; Dupree, Jeff; Franklin, Robin J M.; Casaccia-Bonnefil, Patrizia (24 June 2008). "Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency". Nature Neuroscience. 11 (9): 1024–1034. doi:10.1038/nn.2172. PMC 2656679. PMID 19160500.
- ^ Emery, B. (4 November 2010). "Regulation of Oligodendrocyte Differentiation and Myelination". Science. 330 (6005): 779–782. doi:10.1126/science.1190927. PMID 21051629.

