User:Mr. Ibrahem/Desipramine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norpramin, Pertofrane, others |
| Other names | Desmethylimipramine; Norimipramine; EX-4355; G-35020; JB-8181; NSC-114901[1][2][3] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682387 |
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Tricyclic antidepressant[4] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60–70%[5] |
| Protein binding | 91%[5] |
| Metabolism | Liver (CYP2D6)[6] |
| Elimination half-life | 12–30 hours[5] |
| Excretion | Urine (70%), feces[5] |
| Identifiers | |
| |
| Chemical and physical data | |
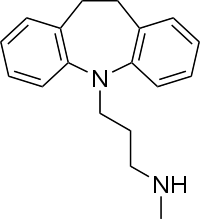
| Formula | C18H22N2 |
| Molar mass | 266.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used to treat depression, panic disorder, and postherpetic neuralgia.[7] While benefits may be seen within 5 days, up to 3 weeks may be required for full effects.[7]
Common side effects include dry mouth, constipation, blurry vision, low blood pressure with standing, sleepiness, and weakness.[7] Other side effects may include suicide, mania, arrythmias, and seizures.[7] Safety in pregnancy is unclear.[7] How it works is unclear, but is believed to involve effects on serotonin and norepinephrine.[7]
Desipramine was approved for medical use in the United States in 1964.[7] It is available as a generic medication.[4] In the United States 30 tablets of 100 mg costs about 25 USD as of 2021.[8] It has been widely used.[4]
References[edit]
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 363–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 304–. ISBN 978-3-88763-075-1.
- ^ "Desipramine - Drugs.com". drugs.com. Archived from the original on 2018-08-30. Retrieved 2021-02-10.
- ^ a b c "Desipramine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 23 December 2021.
- ^ a b c d Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 588–. ISBN 978-1-60913-345-0. Archived from the original on 16 December 2019. Retrieved 10 February 2021.
- ^ Sallee, FR; Pollock, BG (May 1990). "Clinical pharmacokinetics of imipramine and desipramine". Clinical Pharmacokinetics. 18 (5): 346–64. doi:10.2165/00003088-199018050-00002. PMID 2185906. S2CID 37529573.
- ^ a b c d e f g h i "Desipramine Monograph for Professionals". Drugs.com. Archived from the original on 27 January 2021. Retrieved 23 December 2021.
- ^ "Desipramine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 23 December 2021.
