User:Mr. Ibrahem/Dextromethorphan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Robitussin, Delsym, DM, DexAlone, Duract, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682492 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 11%[1] |
| Metabolism | Liver enzymes: major CYP2D6, minor CYP3A4, and minor CYP3A5 |
| Elimination half-life | 2–4 hours (extensive metabolizers); 24 hours (poor metabolizers)[2] |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
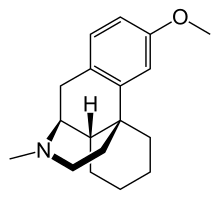
| Formula | C18H25NO |
| Molar mass | 271.404 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 111 °C (232 °F) |
| |
| |
| | |
Dextromethorphan is a medication often used in over-the-counter cough medicines.[3] In cough medicine it may be combined with paracetamol (acetaminophine), chlorpheniramine, pseudoephedrine, or guaifenesin.[3] There is no clear evidence of benefit in children under six to twelve years of age with some evidence of harm; thus such use is not recommended.[3][4] It is taken by mouth.[3]
Side effects may include nausea, sleepiness, and dizziness.[3] Serious side effects may include serotonin syndrome and abuse.[3] While safety in pregnancy and breastfeeding has not been studied, such use is believed to be okay.[5][6] It is in the morphinan class of medications and acts within the brain to suppress coughing.[3][4]
Dextromethorphan was patented in 1949 and approved for medical use in 1953.[7] Dextromethorphan is also used recreationally.[3] At high doses dextromethorphan acts as a dissociative hallucinogen and may result in seizures.[8] A number of other uses for dextromethorphan are being studied.[9]
References[edit]
- ^ Kukanich B, Papich MG (October 2004). "Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs". Journal of Veterinary Pharmacology and Therapeutics. 27 (5): 337–41. doi:10.1111/j.1365-2885.2004.00608.x. PMID 15500572.
- ^ "Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 31 March 2019. Retrieved 15 April 2014.
- ^ a b c d e f g h "Dextromethorphan Hydrobromide Monograph for Professionals". Drugs.com. Archived from the original on 6 November 2020. Retrieved 5 October 2020.
- ^ a b BNF 79. London: Pharmaceutical Press. March 2020. p. 305. ISBN 978-0857113658.
- ^ "Dextromethorphan Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 5 October 2020.
- ^ "Dextromethorphan". Drugs and Lactation Database (LactMed). 2006. PMID 30000516. Archived from the original on 2021-08-27. Retrieved 2020-10-05.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 527. ISBN 9783527607495. Archived from the original on 2021-08-29. Retrieved 2020-09-06.
- ^ Abuse, National Institute on Drug (NaN). "What Are the Effects of Common Dissociative Drugs on the Brain and Body?". National Institute on Drug Abuse. Archived from the original on 10 October 2020. Retrieved 5 October 2020.
{{cite web}}: Check date values in:|date=(help) - ^ Oh, SR; Agrawal, S; Sabir, S; Taylor, A (January 2020). "Dextromethorphan". PMID 30855804.
{{cite journal}}: Cite journal requires|journal=(help)
