User:Mr. Ibrahem/Milrinone
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601020 |
| Routes of administration | IV only |
| Drug class | Phosphodiesterase 3 inhibitor[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (as IV bolus, infusion) |
| Protein binding | 70 to 80% |
| Metabolism | Liver (12%) |
| Elimination half-life | 2.3 hours (mean, in CHF) |
| Excretion | Urine (85% as unchanged drug) within 24 hours |
| Identifiers | |
| |
| Chemical and physical data | |
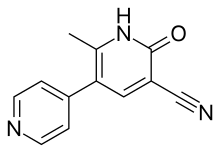
| Formula | C12H9N3O |
| Molar mass | 211.224 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.344 g/cm3 |
| Melting point | 315 °C (599 °F) |
| |
| |
| (verify) | |
Milrinone, sold under the brand name Primacor, is a medication used to treat heart failure.[2] It may be used short term in those in who other medications are not sufficient.[2] It is given by injection into a vein.[2]
Common side effects include ventricular arrhythmia, low blood pressure, and headache.[2] Other side effects may include low potassium and long term use may increase death.[2] It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decreases vascular resistance.[1]
Milrinone was approved for medical use in the United States in 1987.[2] It is available as a generic medication.[1] In the United Kingdom 10 mg cost the NHS about £20 as of 2021.[1] In the United States this amount costs about 5 USD.[3]
References[edit]
- ^ a b c d BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 210. ISBN 978-0857114105.
- ^ a b c d e f g h "Milrinone Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 18 November 2021.
- ^ "Milrinone Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 18 November 2021.
