User:Mr. Ibrahem/Ramelteon
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rozerem, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605038 |
| License data | |
| Addiction liability | None[1] |
| Routes of administration | By mouth |
| Drug class | Melatonin receptor agonist[2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 1.8% |
| Protein binding | ~82% |
| Metabolism | Liver (CYP1A2-mediated) |
| Elimination half-life | 1–2.6 hours |
| Excretion | Kidney (84%) and fecal (4%) |
| Identifiers | |
| |
| Chemical and physical data | |
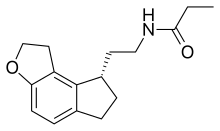
| Formula | C16H21NO2 |
| Molar mass | 259.349 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ramelteon, sold under the brand name Rozerem among others, is a medication used for trouble falling asleep.[3] It is taken by mouth, half an hour before bed.[3] There is no limitation on duration of use; though long term effectiveness is unclear.[4][2]
Common side effects include sleepiness, dizziness, nausea, and worsened trouble sleeping.[3] Other side effects may include anaphylaxis, abnormal thinking, and depression.[3] Effects on pregnancy and breastfeeding are unclear.[5] It is a melatonin receptor agonist and is expected to work similar to melatonin.[2]
Ramelteon was discovered in 1996 and approved for medical use in the United States in 2005.[4][1] It was declined approval in Europe in 2008 due to unclear effectiveness.[2] In the United States it is available as a generic medication and costs about 60 USD per month as of 2021.[6] It is not a controlled substance.[1]
References[edit]
- ^ a b c d "Ramelteon Monograph for Professionals". Drugs.com. Archived from the original on 25 August 2019. Retrieved 15 October 2021.
- ^ a b c d "Ramelteon: Withdrawal of the marketing authorisation application". Archived from the original on 16 October 2021. Retrieved 15 October 2021.
- ^ a b c d e f "Rozerem- ramelteon tablet, film coated". DailyMed. 28 December 2018. Archived from the original on 26 March 2021. Retrieved 13 April 2020.
- ^ a b Neubauer DN (February 2008). "A review of ramelteon in the treatment of sleep disorders". Neuropsychiatric Disease and Treatment. 4 (1): 69–79. doi:10.2147/ndt.s483. PMC 2515902. PMID 18728808.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Ramelteon (Rozerem) Use During Pregnancy". Drugs.com. Archived from the original on 24 January 2021. Retrieved 15 October 2021.
- ^ "Ramelteon Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 31 October 2016. Retrieved 15 October 2021.
