User:Mr. Ibrahem/Rimexolone
 | |
| Clinical data | |
|---|---|
| Trade names | Vexol |
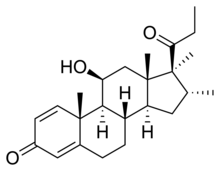
| Other names | Trimexolone; Org 6216; 11β-Hydroxy-16α,17α,21-trimethylpregna-1,4-dien-3,20-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606003 |
| Routes of administration | Eye drops |
| Drug class | Corticosteroid |
| Pharmacokinetic data | |
| Elimination half-life | estimated 1–2 hours |
| Excretion | >80% faeces |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C24H34O3 |
| Molar mass | 370.533 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rimexolone, sold under the brand name Vexol, is a steroid medication used to treat inflammation of the eye.[1] This includes anterior uveitis and following surgery of the eye.[1] It is used as an eye drop.[1]
Common side effects include increased intraocular pressure, blurry vision, eye pain, redness, and a runny nose.[1] Other complications can include fungal infection of the cornea.[1]
Rimexolone was approved for medical use in the United States in 1994.[1] In the United States a 5 ml bottle costs about 100 USD as of 2021.[2] It is no longer available in the United Kingdom as of 2019.[3]
References[edit]
- ^ a b c d e f g h "Rimexolone Monograph for Professionals". Drugs.com. Archived from the original on 20 January 2021. Retrieved 17 October 2021.
- ^ "Vexol Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 17 October 2021.
- ^ "Rimexolone eye drops. Rimexolone antibiotic eye drops - Patient". patient.info. Archived from the original on 13 August 2021. Retrieved 17 October 2021.
