User:Mr. Ibrahem/Zafirlukast
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Accolate, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697007 |
| Pregnancy category |
|
| Routes of administration | By mouth[1] |
| Drug class | Leukotriene receptor antagonist[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | >99% (albumin)[2] |
| Metabolism | Liver (CYP2C9-mediated) |
| Metabolites | hydroxylated metabolites[2] |
| Onset of action | < 2 weeks[1] |
| Elimination half-life | 10 hours |
| Excretion | Fecal[2] |
| Identifiers | |
| |
| Chemical and physical data | |
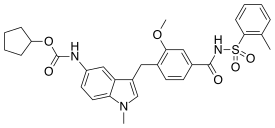
| Formula | C31H33N3O6S |
| Molar mass | 575.68 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 138 to 140 °C (280 to 284 °F) |
| |
| |
| (verify) | |
Zafirlukast, sold under the brand Accolate among others, is a medication used for the long term treatment of asthma and allergic rhinitis.[1] It is less preferred to inhaled steroids for asthma.[1] It is taken by mouth.[1] Benefits may take up to two weeks to occur.[1]
Common side effects include headache and stomach upset.[1] Severe side effects may include liver problems and potentially Churg-Strauss syndrome.[1] Use is likely safe in pregnancy.[1] It is a leukotriene receptor antagonist (LTRA) and works by decreasing airway mucous, swelling, bronchoconstriction, and inflammation.[1]
Zafirlukast was approved for medical use in the United States in 1996.[1] It is approved in some but not all parts of Europe.[3] In the United States it costs about 34 USD per month as of 2021.[4]
References[edit]
- ^ a b c d e f g h i j k l m n "Zafirlukast". Archived from the original on 3 August 2020. Retrieved 4 August 2021.
- ^ a b c d "ACCOLATE (zafirlukast) Package Insert" (PDF). www.accessdata.fda.gov. AstraZeneca LP. Archived (PDF) from the original on 20 January 2017. Retrieved 29 November 2017.
- ^ "List of nationally authorised medicinal products Active substance: Zafirlukast" (PDF). Archived (PDF) from the original on 27 August 2021. Retrieved 4 August 2021.
- ^ "Zafirlukast Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 31 January 2021. Retrieved 4 August 2021.
