User:Msullivan31/Legionella pneumophila
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
| Msullivan31/Legionella pneumophila | |
|---|---|
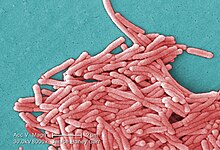
| |
| Colorized scanning electron micrograph image of L. pneumophila | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Legionellales |
| Family: | Legionellaceae |
| Genus: | Legionella |
| Species: | L. pneumophila
|
| Binomial name | |
| Legionella pneumophila Brenner DJ, Steigerwalt AG, McDade JE 1979
| |
Article Draft[edit]
L. pneumophila is a Gram-negative, non-encapsulated, aerobic bacillus with a single, polar flagellum often characterized as being a coccobacillus. It is aerobic and unable to hydrolyse gelatin or produce urease. It is also non-fermentative. L. pneumophila is neither pigmented nor does it autofluoresce. It is oxidase- and catalase-positive, and produces beta-lactamase. L. pneumophila colony morphology is gray-white with a textured, cut-glass appearance; it also requires cysteine and iron to thrive. It grows on yeast extract agar in "opal-like" colonies.
to
L. pneumophila is often characterized as being a coccobacillus. It is a Gram-negative,aerobic bacteria unable to hydrolyse gelatin or produce urease. It is also non-fermentative. L. pneumophila is neither pigmented nor does it autofluoresce. It is oxidase- and catalase-positive, and produces beta-lactamase. L. pneumophila colony morphology is gray-white with a textured, cut-glass appearance; it also requires cysteine and iron to thrive. It grows on yeast extract agar as well as in moist environments, such as tap water, in "opal-like" colonies.
Detection section that randomly popped up whoop whoop:
Antisera have been used both for slide agglutination studies and for direct detection of bacteria in tissues using immunofluorescence via fluorescent-labelled antibody. Specific antibody in patients can be determined by the indirect fluorescent antibody test. ELISA and microagglutination tests have also been successfully applied.[1]
Legionella stains poorly with Gram stain, stains positive with silver, and is cultured on charcoal yeast extract with iron and cysteine.[citation needed] A consistent method that has been used to detect the disease is the urine antigen test. This test identifies specific antigens excreted in the urine that may trigger an immune response.[2]
Ecology and reservoirs -Maddie editing[edit]

L. pneumophila is a bacterium that can be found in numerous different environmental conditions. It can preside in temperatures ranging from 0-63 °C, a pH range of 5.0-8.5, and in dissolved oxygen levels of 0.2-15.0 mg/liter.[3]
In the environment, it can be found in freshwater environments within this wide range of temperatures. Although it can be found in this wider range, it only multiplies within a temperature of 25 and 42 °C.[3] With their ability to preside in water, they can also resist chlorination of water and pass into water control systems easily. [3] With this ability to infiltrate water systems, they can form biofilms in the walls of pipes which can lead to this bacterium being aerosolized through faucets, showers, sprinklers, and other fixtures, leading to infection after prolonged exposure. [4] With the main cause of L. pneumophila contamination being the water supply network, this has caused L. pneumophila to grow and proliferate in places such as: cooling towers, water systems of hospitals, hotels, and cruise ships. [3]
As a facultative intracellular parasite, L. pneumophila can invade and replicate inside amoebae in the environment, especially within the species of the genera Acanthamoeba and Naegleria, which can thus serve as a reservoir for L. pneumophila. These hosts will then provide protection against unfavorable physical and chemical conditions, such as chlorination. [5]
Biofilms
Biofilms are specialized, surface attachment communities that can consist of one microbe, or multiple different ones, ranging from bacteria, algae, and protozoa. Biofilms on plumbing systems and in water distribution systems is where a lot of L. pneumophila can survive. [3] Between 2009 and 2010, L. pneumophila contributed to 58% of the total waterborne disease outbreaks associated with drinking water in the United States, causing an increase in research surrounding L. pneumophila biofilms and proliferation. [6] Material also plays a role in biofilm proliferation. In water piping, L. pneumophila was more commonly found in plastic pipes at 40°C, rather than a copper pipe, which actually inhibited growth.[3]
Free-Living Protozoa
Legionella is a species that is known to infect and multiply within species of free-living amoeba. We know of at least 20 different species of free-living amoeba that support the intracellular replication of L. pneumophila. [7] This bacterium can infect and survive within the amoeba genera which includes: Acanthamoeba, Vermamoeba and Naegleria. L. pneumophila are surrounded by a amoeba-resistant cyst while residing inside the amoeba, allowing them to survive harsh environmental conditions such as chlorine, which is commonly used in water treatment systems. [8]
Although it is known that free-living amoeba play an important role in the ecology of L. pneumophila, there is little data on how these amoebas interact with other amoebas, and how this affects L. pneumophila overall. [7]
Frequency of occurrence[edit]
In the United States, approximately 3 infections with L. pneumophila appear per 100,000 people per year. The infections peak in the summer. Within endemic regions, about 4% to 5% of pneumonia cases are caused by L. pneumophila.
L. pneumopila is the primary (>90%) causative organism for Legionnaires disease.[9] Roughly 2 per 100,000 people are infected with Legionnaires disease each year in the EU.[10] L. pneumopila often infects individuals through poor quality water sources. Approximately 20% of reported Legionnaires disease cases come from healthcare, senior living, or travel facilities that have been exposed to water contaminated with L. pneumopila. [9]There may also be an increased risk of contracting L. pneumopila from private wells, as they are often unregulated and not as rigorously disinfected as municipal water systems.[11] Several large outbreaks of Legionnaire's Disease have come from public baths such as spas and hot tubs due to the temperature range of the water being ideal for Legionella growth.[12][13]
Pathogenesis[edit]

In humans, L. pneumophila invades and replicates inside macrophages. The internalization of the bacteria can be enhanced by the presence of antibody and complement, but is not absolutely required. Internalization of the bacteria appears to occur through phagocytosis. However, L. pneumophila is also capable of infecting non-phagocytic cells through an unknown mechanism. A rare form of phagocytosis known as coiling phagocytosis has been described for L. pneumophila, but this is not dependent on the Dot/Icm (intracellular multiplication/defect in organelle trafficking genes) bacterial secretion system and has been observed for other pathogens. Once internalized, the bacteria surround themselves in a membrane-bound vacuole that does not fuse with lysosomes that would otherwise degrade the bacteria. In this protected compartment, the bacteria multiply.
Virulence Factors[edit]
L. pneumophila possesses a unique lipopolysaccharide (LPS) structure that is highly hydrophobic due to it being densely packed with branched fatty acids, and elevated levels of O-acetyl and N-acetyl groups. This structure helps prevent interaction with a common LPS immune system co-receptor, CD14. [14] There is also a correlation between an LPS with a high molecular-weight and the inhibition of phagosome-lysosome fusion.[14] L. pneumophila produces pili of varying lengths. The two pili proteins: PilE and Prepilin peptidase (PilD) are responsible for the production of type IV pili and subsequently intracellular proliferation.[15] L. pneumophila exhibits a singular, polar flagellum that is used for cell motility, adhesion, host invasion, and biofilm formation.[14] The same regulators that control flagellation also control lysosome avoidance and cytotoxicity.[14] The Macrophage Infectivity Potentiator (MIP) is another key component of host cell invasion and intracellular replication. MIP displays peptidyl–prolyl cis/trans isomerase (PPIase) activity which is crucial for survival within the macrophage, along with transmigration across the lung epithelial barrier.[14][15]
Metabolism Overview and Nutrient Acquisition[edit]
Overall, every genome that has been reported of L. pneumophila utilizes gylcolysis, the Enter-Doudoroff (ED) pathway, the pentose phosphate pathway (PP), and the citric acid cycle (TCA).[16] Although L. pneumophila can also perform gluconeogenesis, it does not have the needed genes to encode for 1,6-biphosphatases. Therefore, other enzymes are used to complete gluconeogenesis. One enzyme used instead is fructose 6-phosphate aldolase.[16] This trend is also present when it comes to the PP pathway which can occur without substrates such as 6-phosphogluconate dehydrogenase. [16] The ED and PP pathways are the main pathways for glucose metabolism in this organism. Along with these pathways, serine was found to be a major nutrient due to its ability to be turned into pyruvate, which is an important intermediate in metabolic pathways in L. pneumophila.[16]
Although glucose metabolism is used, it is not one of the main synthesis pathways within the organism. While utilizing media with glucose, growth of L. pneumophila did not increase and carbohydrates were not considered an important carbon source within L. pneumophila. With more recent discoveries, it has been made known that glucose can act as a co-substrate only under certain conditions, as this microbe utilizes amino acids more frequently and efficiently. [16]
Nutrient Acquisition
Legionella is auxotrophic for seven amino acids: cysteine, leucine, methionine, valine, threonine, isoleucine, and arginine. Once inside the host cell, Legionella needs nutrients to grow and reproduce. Inside the vacuole, nutrient availability is low; the high demand of amino acids is not covered by the transport of free amino acids found in the host cytoplasm. To improve the availability of amino acids, the parasite promotes the host mechanisms of proteasomal degradation. This process in L. pneumophila includes the SCF1 ubiquitin ligase and the AnkB F-Box effector, which is farnesylated by the activity of three host enzymes localized in the membrane of the LCV: farnesyltransferase, Ras-converting enzyme-1 protease, and ICMT. Farnesylation allows AnkB to get anchored into the cytoplasmic side of the vacuole. SCF1 and AnkB interact with each other to degrade Lys-linked polyubiquitinated proteins.[17] This generates an excess of free amino acids in the cytoplasm of L. pneumophila-infected cells that can be used for intravacuolar proliferation of the parasite.
The K48-linked polyubiquitination is a marker for proteasomal degradation that releases 2 to 24-amino-acid-long peptides, which are quickly degraded to amino acids by various oligopeptidases and aminopeptidases present in the cytoplasm. Amino acids are imported into the LCV through various amino acid transporters such as the neutral amino acid transporter B(0).[18]
The amino acids are the primary carbon and energy source of L. pneumophila, that have almost 12 classes of ABC-transporters, amino acid permeases, and many proteases, to exploit it. The imported amino acids are used by L. pneumophila to generate energy through the TCA cycle (Krebs cycle) and as sources of carbon and nitrogen. Because the amino acid degradation acts as the main carbon source for L. pneumophila, this microbe does not rely as heavily on glucose. Despite this, L. pneumophila does contain multiple amylases, such as LamB, which hydrolyzes polysaccharides into glucose monomers for metabolism. The loss of LamB can result in severe growth issues for L. pneumophila.[19]
However, promotion of proteasomal degradation for the obtention of amino acids and the hydrolyzation of polysaccharides may not be the only virulence strategies to obtain carbon and energy sources from the host. Type II–secreted degradative enzymes may provide an additional strategy to generate carbon and energy sources.[20] L. pneumophila is the only known intracellular pathogen to have a Type II Secretion System (secretome). In Type II Secretion, proteins are first translocated across the inner membrane into the periplasmic space. This process is mediated by either the Sec or Tat pathway. Soon after, the same proteins are then transported through a specific pore in the outer membrane to the exterior of the cell. This secretome is believed to have as many as 60 proteins incorporated into the system. [20]
Drug targets and Treatment[edit][edit]
Macrolides (azithromycin or clarithromycin) or fluoroquinolones (levofloxacin or moxifloxacin) are the standard treatment for Legionella pneumonia in humans, with levofloxacin being considered first line with increasing resistance to azithromycin. Two studies support superiority of levofloxacin over macrolides, although not FDA approved.
Several enzymes in the bacteria have been proposed as tentative drug targets. For example, enzymes in the iron uptake pathway have been suggested as important drug targets. Further, a cN-II class of IMP/GMP specific 5´-nucleotidase which has been extensively characterized kinetically. The tetrameric enzyme shows aspects of positive homotropic cooperativity, substrate activation and presents a unique allosteric site that can be targeted to design effective drugs against the enzyme and thus, the organism. Moreover, the enzyme is distinct than its human counterpart making it an attractive target for drug development.
Effective antibiotic treatment for Legionella pneumonia includes fluoroquinolones (levofloxacin or moxifloxacin) or macrolides ( preferably azithromycin).[21] There has been no significant difference found between using a fluoroquinolone or a macrolide to treat Legionella pneumonia.[21] Combination treatments with rifampicin are being tested as a response to antibiotic resistance during monotreatments, though its effectiveness remains uncertain.[21]
These antibiotics work best because L. pneumophila is an intracellular pathogen.[22] Fluoroquinolones and macrolides have great intracellular activity and are able to penetrate into Legionella-infected cells. The Infectious Diseases Society of America recommends 5-10 days of treatment for levofloxacin and 3-5 days of treatment for azithromycin, however patients that are immunocompromised or have a severe disease may require an extended course of treatment.[22]
good article if anyone else wants to contribute to this part: https://doi.org/10.1002/1873-3468.12326
Lead[edit]
Peer Review -Methanococcus maripaludis group[edit]
Very informative sections of content but some of the formatting was confusing because you had sections about drug and treatment of infections but not much content on the mechanisms of infection first before reading drug and treatment section.
- - L. pneuomphila consists of 90% of Legionaire's cases --> possibly add more relevance
- L. pneumophila is a bacterium that can be found in numerous different environmental conditions. It can preside in temperatures ranging from 0-63 °C, a pH range of 5.0-8.5, and in dissolved oxygen levels of 0.2-15.0 mg/liter. --> i like that you added more specific details to their habitat. maybe specify mesophile, thermophile, etc.?
- Biofilms on plumbing systems and in water distribution systems is where a lot of L. pneumophila can survive. --> be careful with grammar (is --> are) but i like the specifications about their most common habitats and how they may interact with humans
-- combined drug targets and treatment --> i dont think you should spend a lot of time on this (not included in rubric)
-Great start! maybe add a heading about metabolism (how they gain carbon, energy, electrons)
References[edit]
- ^ Conway de Macario, E; Macario, A J; Wolin, M J (January 1982). "Specific antisera and immunological procedures for characterization of methanogenic bacteria". Journal of Bacteriology. 149 (1): 320–328. ISSN 0021-9193. PMID 6172417.
- ^ Viasus, Diego; Gaia, Valeria; Manzur-Barbur, Carolina; Carratalà, Jordi (2022-6). "Legionnaires' Disease: Update on Diagnosis and Treatment". Infectious Diseases and Therapy. 11 (3): 973–986. doi:10.1007/s40121-022-00635-7. ISSN 2193-8229. PMC 9124264. PMID 35505000.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f Hames-Kocabas, E (2010). "Legionella Pneumophila : From Environment to Disease". NOVA Science Publishers: 5–8 – via ebscohost.
- ^ Abdel-Nour, Mena (2013). "Biofilms: The Stronghold of Legionella pneumophila". Int J Mol Sci. 11: 21660–21675 – via PubMed.
- ^ Greub, G (Nov 2003). "Morphology of Legionella pneumophila according to their location within Hartmanella vermiformis". Research in Microbiology. 154 (9): 619–21 – via PubMed.
- ^ Shen, Y (2015). "Role of Biofilm Roughness and Hydrodynamic Conditions in Legionella pneumophila Adhesion to and Detachment from Simulated Drinking Water Biofilms". Environmental Science & Technology. 49 (7): 4274–4282 – via ACS.org.
- ^ a b Dey, Rafik (2020). "Impact of inter-amoebic phagocytosis on the L. pneumophila growth". FEMS Microbiology Letters. 367 (18) – via Oxford Academic.
- ^ Muchesa, P (2018). "Detection of amoeba-associated Legionella pneumophila in hospital water networks of Johannesburg". Southern African Journal of Infectious Diseases. 33 (3): 72–75 – via SAJID.
- ^ a b Donohue, Maura J.; Pham, Maily; Brown, Stephanie; Easwaran, Kaveri M.; Vesper, Stephen; Mistry, Jatin H. (2023-06-30). "Water quality influences Legionella pneumophila determination". Water Research. 238: 119989. doi:10.1016/j.watres.2023.119989. ISSN 0043-1354. PMC 10351031. PMID 37137207.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Lupia, Tommaso; Corcione, Silvia; Shbaklo, Nour; Rizzello, Barbara; De Benedetto, Ilaria; Concialdi, Erika; Navazio, Anna Sara; Penna, Maurizio; Brusa, Maria Teresa; De Rosa, Francesco Giuseppe (2023-02). "Legionella pneumophila Infections during a 7-Year Retrospective Analysis (2016–2022): Epidemiological, Clinical Features and Outcomes in Patients with Legionnaires' Disease". Microorganisms. 11 (2): 498. doi:10.3390/microorganisms11020498. ISSN 2076-2607. PMC 9965988. PMID 36838463.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Mapili, K.; Pieper, K. J.; Dai, D.; Pruden, A.; Edwards, M. A.; Tang, M.; Rhoads, W. J. (2020-04-01). "Legionella pneumophila occurrence in drinking water supplied by private wells". Letters in Applied Microbiology. 70 (4): 232–240. doi:10.1111/lam.13273. ISSN 1472-765X.
- ^ Kuroki, Toshiro; Amemura-Maekawa, Junko; Ohya, Hitomi; Furukawa, Ichiro; Suzuki, Miyuki; Masaoka, Tomoka; Aikawa, Kastuhiro; Hibi, Kazumi; Morita, Masatomo; Lee, Ken-ichi; Ohnishi, Makoto; Kura, Fumiaki (2017-02). "Outbreak of Legionnaire's Disease Caused by Legionella pneumophila Serogroups 1 and 13". Emerging Infectious Diseases. 23 (2): 349–351. doi:10.3201/eid2302.161012. ISSN 1080-6040. PMC 5324795. PMID 28098535.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Donovan, Catherine V.; MacFarquhar, Jennifer K.; Wilson, Erica; Sredl, Megan; Tanz, Lauren J.; Mullendore, Jennifer; Fleischauer, Aaron; Smith, Jessica C.; Lucas, Claressa; Kunz, Jasen; Moore, Zack (2023-03-27). "Legionnaires' Disease Outbreak Associated With a Hot Tub Display at the North Carolina Mountain State Fair, September 2019". Public Health Reports: 003335492311591. doi:10.1177/00333549231159159. ISSN 0033-3549.
- ^ a b c d e Shevchuk, Olga; Jäger, Jens; Steinert, Michael (2011). "Virulence Properties of the Legionella Pneumophila Cell Envelope". Frontiers in Microbiology. 2. doi:10.3389/fmicb.2011.00074/full. ISSN 1664-302X.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Talapko, Jasminka; Frauenheim, Erwin; Juzbašić, Martina; Tomas, Matej; Matić, Suzana; Jukić, Melita; Samardžić, Marija; Škrlec, Ivana (2022-01-24). "Legionella pneumophila—Virulence Factors and the Possibility of Infection in Dental Practice". Microorganisms. 10 (2): 255. doi:10.3390/microorganisms10020255. ISSN 2076-2607. PMC 8879694. PMID 35208710.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Eisenreich, Wolfgang (2016). "The life stage-specific pathometabolism of Legionella pneumophila". FEBS Letters. 590 (21): 3868–3886 – via FEBS PRESS.
- ^ Price, Christopher T. D.; Richards, Ashley M.; Abu Kwaik, Yousef (2014-08-26). "Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila". Frontiers in Cellular and Infection Microbiology. 4: 111. doi:10.3389/fcimb.2014.00111. ISSN 2235-2988. PMC 4143614. PMID 25207263.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Price, Christopher T. D.; Richards, Ashley M.; Abu Kwaik, Yousef (2014-08-26). "Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila". Frontiers in Cellular and Infection Microbiology. 4: 111. doi:10.3389/fcimb.2014.00111. ISSN 2235-2988. PMC 4143614. PMID 25207263.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Best, Ashley; Price, Christopher; Ozanic, Mateja; Santic, Marina; Jones, Snake; Abu Kwaik, Yousef (2018-04-20). "A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae". Scientific Reports. 8 (1): 6340. doi:10.1038/s41598-018-24724-1. ISSN 2045-2322.
- ^ a b Mintz, C. S. (1999-12). "Gene transfer in Legionella pneumophila". Microbes and Infection. 1 (14): 1203–1209. doi:10.1016/s1286-4579(99)00241-5. ISSN 1286-4579. PMID 10580276.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Viasus, Diego; Gaia, Valeria; Manzur-Barbur, Carolina; Carratalà, Jordi (2022-06). "Legionnaires' Disease: Update on Diagnosis and Treatment". Infectious Diseases and Therapy. 11 (3): 973–986. doi:10.1007/s40121-022-00635-7. ISSN 2193-8229.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Cunha, Burke A; Burillo, Almudena; Bouza, Emilio (2016-01). "Legionnaires' disease". The Lancet. 387 (10016): 376–385. doi:10.1016/s0140-6736(15)60078-2. ISSN 0140-6736.
{{cite journal}}: Check date values in:|date=(help)
