User:Ogletreerd
__FORCETOC__
Genome Engineering: Multiplex Automated Genomic Engineering (MAGE)[edit]

Chemically synthesized single-stranded DNA (ssDNA) and a pool of oligionucleotides are transformed into cells and replace endogenous sequences thereby creating genetic modifications. The cyclical process involves transformation of ssDNA (by electroporation) followed by outgrowth, during which bacteriophage recombination proteins mediate homologous recombination of ssDNAs with their genomic targets. Modified cells are identified by selective phenotypic markers by plating the cells on selective media. Each cycle ultimately takes 2.5 hours to process, with additional time required to grow isogenic cultures and characterize mutations. By iteratively introducing libraries of mutagenic ssDNAs targeting multiple sites, MAGE can generate combinatorial genetic diversity in a cell population. There can be up to 50 genome edits, from single nucleotide base pairs to whole genome or gene networks simultaneously with results in a matter of days[1].
TALENs[edit]

Transcription activator-like effector nucleases (TALENs) are artificial restriction enzymes generated by fusing specific DNA-binding domains to a non-specific DNA cleaving domain (usually FokI). The DNA binding domains, which can be designed to bind any desired DNA sequence, comes from TAL effectors, DNA-binding proteins excreted by plant pathogens such as Xanthomanos spp. Tal effectors consist of repeated domains, each containing a highly conserved sequence of 34 amino acids, and recognize a single DNA nucleotide. The nuclease can create double strand breaks at the target site that can be repaired by error-prone non-homologous end-joining (NHEJ), resulting in gene disruptions through the introduction of small insertions or deletions TALENs are used in a similar way as zinc finger nucleases, and have three advantages in targeted mutagenesis: (1) DNA binding specificity is higher, (2) off-target effects are lower, and (3) construction of DNA-binding domains is easier.
Genome Evolution: History[edit]
Prokaryote genomes[edit]

Evolution of prokaryotes led to more complex living organisms, and scientist theorized that this process resulted from a more efficient acquisition of food. Although aracheabacteria were the earliest organisms, bacteria make up the majority of the prokaryotes and represent the diversity of life. As bacteria modified structures to expand their territory and tolerance, they changed into newer species of bacteria with diverse structures and functions.
In terms of organization, bacterial and archaeal genomes have similar genetic organization, typically with a single origin of replication. Additionally, their genome complexity and functions are very similar. Genetic changes may lead to an increase or loss of genomic complexity, e.g. due to adaptive genome streamlining and purifying selection[2].
Protein-Protein Interactions[edit]
Text Mining[edit]
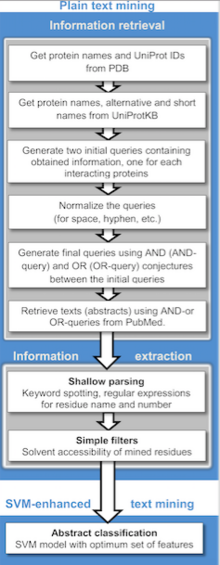
Publicly available information from biomedical research is readily accessible through the internet and is becoming a powerful resource for predicting protein-protein interactions and protein docking. Text mining is much less costly and less time-consuming compared to other high-throughput techniques. Currently, these methods generally detect binary relations between interacting protein from individual sentences using machine learning and rule/pattern based information extraction and machine learning approaches[3]. A wide variety of text mining predicting PPIs applications are available for public use, as well as repositories which often stores manually validated and/or computationally predicted PPIs. The principal stages of text mining divides the implementation into two stages: information retrieval, where literature abstracts containing names of either or both proteins complexes are selected and information extraction, where detecting occurrences of residues are retrieved. The extraction is automated by searching for co-existing sentences, abstracts or paragraphs within textual context.
There are also studies using phylogenetic profiling, basing their functionalities on the theory that proteins involved in common pathways co-evolve in a correlated fashion across a large number of species. More complex text mining methodologies use advanced dictionaries and generate networks by Natural Language Processing (NLP) of text, considering gene names as nodes and verbs as edges. Other developments involve kernel methods to predict protein interactions[4].

X-ray Crystallization: Protein Crystallization[edit]
Microbatch[edit]
Other approaches involves, crystallizing proteins under oil, where aqueous protein solutions are dispensed under liquid oil, and water evaporates through the layer of oil. Different oils have different evaporation permeabilities, therefore yielding changes in concentration rates from different percipient/protein mixture. The technique relies on bringing the protein directly into the nucleation zone by mixing protein with the appropriate amount of percipient to prevent the diffusion of water out of the drop.
References[edit]
- ^ Wang, Harris H.; Isaacs, Farren J.; Carr, Peter A.; Sun, Zachary Z.; Xu, George; Forest, Craig R.; Church, George M. (2009-08-13). "Programming cells by multiplex genome engineering and accelerated evolution". Nature. 460 (7257): 894–898. doi:10.1038/nature08187. ISSN 0028-0836. PMC 4590770. PMID 19633652.
- ^ Koonin, Eugene V.; Wolf, Yuri I. (2008-12-01). "Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world". Nucleic Acids Research. 36 (21): 6688–6719. doi:10.1093/nar/gkn668. ISSN 0305-1048. PMC 2588523. PMID 18948295.
- ^ Badal, Varsha D.; Kundrotas, Petras J.; Vakser, Ilya A. (2015-12-09). "Text Mining for Protein Docking". PLoS Comput Biol. 11 (12): e1004630. doi:10.1371/journal.pcbi.1004630.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Papanikolaou, Nikolas; Pavlopoulos, Georgios A.; Theodosiou, Theodosios; Iliopoulos, Ioannis (2015-03-01). "Protein–protein interaction predictions using text mining methods". Methods. Text mining of biomedical literature. 74: 47–53. doi:10.1016/j.ymeth.2014.10.026.
