User:Mr. Ibrahem/Fluticasone/salmeterol
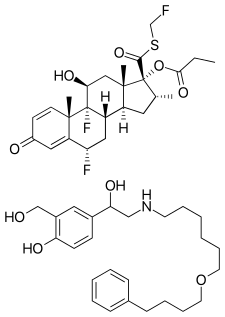 Fluticasone propionate (top) and salmeterol (bottom) | |
| Combination of | |
|---|---|
| Fluticasone propionate | Glucocorticoid |
| Salmeterol | Long-acting β2 agonist (LABA) |
| Clinical data | |
| Trade names | Advair, Seretide, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| MedlinePlus | a699063 |
| Pregnancy category |
|
| Routes of administration | Inhalation |
| Legal status | |
| Legal status | |
| (verify) | |
Fluticasone/salmeterol, sold under the brand name Advair among others, is a combination medication containing fluticasone propionate and salmeterol.[2] It is used in the management of asthma and chronic obstructive pulmonary disease (COPD).[2] It is used by inhaling the medication into the lungs.[2]
Common side effects include thrush, headache, and cough.[3] Serious side effects may include worsening asthma, anaphylaxis, seizures, and heart problems.[3] Safety in pregnancy and breastfeeding is unclear.[4] Fluticasone, a corticosteroid, works by decreasing inflammation while salmeterol, a long-acting beta-adrenoceptor agonist (LABA), works by activating beta-2 adrenergic receptors.[3]
The combination was approved for medical use in the United States in 2000.[3] A generic version was approved in the United States in 2019.[5] A two month supply in the United Kingdom costs the National Health Service about £35 per month as of 2019.[2] In the United States the wholesale cost of this amount is about 89 USD.[6] In 2019, it was the 80th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[7][8]
References[edit]
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 7 September 2020.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 263–264. ISBN 9780857113382.
- ^ a b c d "Fluticasone and Salmeterol inhalation - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 4 March 2019.
- ^ "Fluticasone / salmeterol Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ Office of the Commissioner. "Press Announcements - FDA approves first generic Advair Diskus". www.fda.gov. Retrieved 1 February 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "The Top 300 of 2019". ClinCalc. Retrieved 16 October 2021.
- ^ "Fluticasone; Salmeterol - Drug Usage Statistics". ClinCalc. Retrieved 16 October 2021.
